N-Oxoammonium salt
N-Oxoammonium salts are a class of organic compounds with the formula [R1R2N+=O]X−. The cation [R1R2N+=O] is of interest for the dehydrogenation of alcohols. Oxoammonium salts are diamagnetic, whereas the nitroxide has a doublet ground state. A prominent nitroxide is prepared by oxidation of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, commonly referred to as [TEMPO]+. A less expensive analogue is Bobbitt's salt.
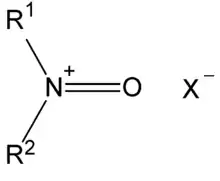
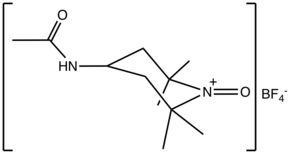
Structure and bonding
Oxoammonium cations are isoelectronic with carbonyls and structurally related to aldoximes (hydroxylamines), and aminoxyl (nitroxide) radicals, with which they can interconvert via a series of redox steps. According to X-ray crystallography, the N-O distance in [TEMPO]BF4 is 1.184 Å, 0.1 Å shorter than the N-O distance of 1.284 Å in the charge-neutral TEMPO. Similarly, the N in [TEMPO]+ is nearly planar, but the O moves 0.1765 Å out of the plane in the neutral TEMPO.[1]
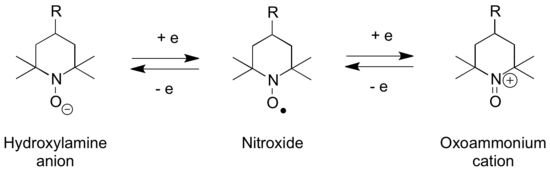
The N-oxoammonium salts are used for oxidation of alcohols to carbonyl groups,[2] as well as other forms of oxoammonium-catalyzed oxidations. The nitroxyl TEMPO reacts via its N-oxoammonium salt.[3]
See also
- Nitrone – structurally related, the N-oxide of an imine
References
- Yonekuta Yasunori, Oyaizu Kenichi, Nishide Hiroyuki (2007). "Structural Implication of Oxoammonium Cations for Reversible Organic One-electron Redox Reaction to Nitroxide Radicals". Chem. Lett. 36 (7): 866–867. doi:10.1246/cl.2007.866.CS1 maint: multiple names: authors list (link)
- Bobbitt, James M.; Brückner, Christian; Merbouh, Nabyl (2010). "Oxoammonium- and Nitroxide-Catalyzed Oxidations of Alcohols". Organic Reactions: 103–424. doi:10.1002/0471264180.or074.02. ISBN 978-0471264187.
- N. E. Leadbeater, J. M. Bobbitt (2014). "TEMPO-Derived Oxoammonium Salts as Versatile Oxidizing Agents" (PDF). Aldrichchimica Acta. 47 (3).
