Neuronal calcium sensor-1
Neuronal calcium sensor-1 (NCS-1) also known as frequenin homolog (Drosophila) (freq) is a protein that is encoded by the FREQ gene in humans.[4] NCS-1 is a member of the neuronal calcium sensor family,[5] a class of EF hand containing calcium-myristoyl-switch proteins.[6]
Function
NCS-1 regulates synaptic transmission,[7] helps control the dynamics of nerve terminal growth,[8][9][7] is critical for some forms of learning and memory in C. elegans[10] and mammals,[11] regulates corticohippocampal plasticity; and enhancing levels of NCS-1 in the mouse dentate gyrus increases spontaneous exploration of safe environments,[11] potentially linking NCS-1 to curiosity.[12]
NCS-1 is a calcium sensor, not a calcium buffer (chelator); thus it is a high-affinity, low-capacity, calcium-binding protein.
Frq can substitute for calmodulin in some situations. It is thought to be associated with neuronal secretory vesicles and regulate neurosecretion.
- It is the Ca2+-sensing subunit of the yeast phosphatidylinositol (PtdIns)-4-OH kinase, PIK1
- It binds to many proteins, some in calcium dependent and some in calcium independent ways, and switches many of the targets "on" (some off).
- Calcineurin (protein phosphatase 2B)
- GRK2 (G-protein-coupled receptor kinase 2)
- D2 dopamine receptor
- IL1RAPL1 (interleukin-1 receptor accessory protein-like 1 protein)
- PI4KIIIβ (type III phosphatidylinositol 4-kinase β)
- IP3 receptor (this activity is inhibited by lithium - a drug used for the treatment of bipolar disorder)[13]
- 3',5'-cyclic nucleotide phosphodiesterases
- ARF1 (ADP Ribosylation factor 1)
- A type (Kv4.3; Shal-related subfamily, member 3) voltage-gated potassium channels
- Nitric oxide synthase
- TRPC5 channel[14]
- Ric8a[15]
- Frq modulates Ca2+ entry through a functional interaction with the α1 voltage-gated Ca2+-channel subunit.[7]
Structure
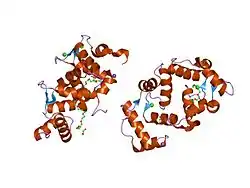
NCS-1 is a globular protein consisting of ten alpha-helices. Four pairs of alpha-helices each form independent 12-amino-acid loops containing a negatively charged calcium binding domain known as an EF-hand. However, only three of these EF hands are functional (the most N-terminal EF-hand does not bind calcium). They could be occupied not only by calcium but also by magnesium and zinc ions.[16] NCS-1 also contains at least two known protein binding domains, and a large surface exposed hydrophobic crevice containing EF-hands three and four. There is a myristoylation motif at the N-terminus that presumably allows NCS-1 to associate with lipid membranes.
Clinical significance
The expression of NCS-1 increases in bipolar disorder and some forms of schizophrenia[17] and decreases in inflammatory bowel disease.[18] NCS-1 has also been linked with Autism.[19] In addition NCS-1 is significant in intelligence in creating curiosity by its function on dopamine D2 receptors in the dentate gyrus, increasing memory for complex tasks. http://www.physorg.com/news172174436.html
History
NCS-1 was originally discovered in Drosophila as a gain-of-function mutation associated with frequency-dependent increases in neurotransmission.[20] A role in neurotransmission was later confirmed in Drosophila using frq null mutants.[7] Work in bovine chromaffin cells demonstrated that NCS-1 is also a modulator of neurotransmission in mammals.[21] The designation 'NCS-1' came from the assumption that the protein was expressed only in neuronal cell types, which is not the case.[22]
References
- GRCh38: Ensembl release 89: ENSG00000107130 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O (April 2001). "Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1)". J. Biol. Chem. 276 (15): 11949–55. doi:10.1074/jbc.M009373200. PMID 11092894.
- Burgoyne RD (2007). "Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling". Nat. Rev. Neurosci. 8 (3): 182–193. doi:10.1038/nrn2093. PMC 1887812. PMID 17311005.
- Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV (2004). "Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function". Trends Neurosci. 27 (4): 203–9. doi:10.1016/j.tins.2004.01.010. PMID 15046879. S2CID 24156457.
- Dason JS, Romero-Pozuelo J, Marin L, Iyengar BG, Klose MK, Ferrus A, Atwood HL (2009). "Frequenin/NCS-1 and the Ca2+-channel {alpha}1-subunit co-regulate synaptic transmission and nerve-terminal growth". Journal of Cell Science. 122 (22): 4109–4121. doi:10.1242/jcs.055095. PMID 19861494.
- Romero-Pozuelo J, Dason JS, Atwood HL, Ferrus A (2007). "Chronic and acute alterations in the functional levels of Frequenins 1 and 2 reveal their roles in synaptic transmission and axon terminal morphology". European Journal of Neuroscience. 26 (9): 2428–2443. doi:10.1111/j.1460-9568.2007.05877.x. hdl:10261/72998. PMID 17970740.
- Hui K, Fei GH, Saab BJ, Su J, Roder JC, Feng ZP (2007). "Neuronal calcium sensor-1 modulation of optimal calcium level for neurite outgrowth". Development. 134 (24): 4479–4489. doi:10.1242/dev.008979. PMID 18039973.
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P (2001). "Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans". Neuron. 30 (1): 241–8. doi:10.1016/S0896-6273(01)00276-8. PMID 11343658. S2CID 9413106.
- Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC (2009). "NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory". Neuron. 63 (5): 643–56. doi:10.1016/j.neuron.2009.08.014. PMID 19755107. S2CID 5321020.
- McDermott, Melissa (September 14, 2009). "Researchers discover the first-ever link between intelligence and curiosity". PHYS ORG. Retrieved 21 September 2012.
- Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE (2006). "Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium". J. Clin. Invest. 116 (6): 1668–74. doi:10.1172/JCI22466. PMC 1459068. PMID 16691292.
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, Levenson R, Beech DJ, Weiss JL (April 2006). "Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth". J. Physiol. 572 (Pt 1): 165–72. doi:10.1113/jphysiol.2005.102889. PMC 1779652. PMID 16469785.
- Romero-Pozuelo J, Dason JS, Mansilla A, Baños-Mateos S, Sardina JL, Chaves-Sanjuán A, Jurado-Gómez J, Santana E, Atwood HL, Hernández-Hernández A, Sánchez-Barrena MJ, Ferrús A (2014). "The guanine-exchange factor Ric8a binds to the Ca2+ sensor NCS-1 to regulate synapse number and neurotransmitter release". Journal of Cell Science. 127 (19): 4246–4259. doi:10.1242/jcs.152603. PMID 25074811.
- Tsvetkov PO, Roman AY, Baksheeva VE, Nazipova AA, Shevelyova MP, Vladimirov VI, Buyanova MF, Zinchenko DV, Zamyatnin AA, Devred F, Golovin AV, Permyakov SE, Zernii EY (2018). "Functional Status of Neuronal Calcium Sensor-1 Is Modulated by Zinc Binding" (PDF). Frontiers in Molecular Neuroscience. 11: 459. doi:10.3389/fnmol.2018.00459. PMC 6302015. PMID 30618610.
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS (2003). "Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients". Proc Natl Acad Sci U S A. 100 (1): 313–7. Bibcode:2003PNAS..100..313K. doi:10.1073/pnas.232693499. PMC 140961. PMID 12496348.
- Lourenssen S, Jeromin A, Roder J, Blennerhassett MG (2002). "Intestinal inflammation modulates expression of the synaptic vesicle protein neuronal calcium sensor-1". Am. J. Physiol. Gastrointest. Liver Physiol. 282 (6): G1097–104. doi:10.1152/ajpgi.00320.2001. PMID 12016136.
- Handley MT, Lian LY, Haynes LP, Burgoyne RD (2010). "Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder". PLOS ONE. 5 (5): e10534. Bibcode:2010PLoSO...510534H. doi:10.1371/journal.pone.0010534. PMC 2866544. PMID 20479890.
- Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R, Mallart A, Galceran J, Canal I, Barbas A, Ferrus A (1993). "Frequenin--a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system". Neuron. 11 (1): 15–28. doi:10.1016/0896-6273(93)90267-U. PMID 8101711. S2CID 30422835.
- McFerran BW, Weiss JL, Burgoyne RD (October 1999). "Neuronal Ca(2+) sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca(2+)-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca(2+) signal transduction". Journal of Biological Chemistry. 274 (42): 30258–65. doi:10.1074/jbc.274.42.30258. PMID 10514519.
- Nef S, Fiumelli H, de Castro E, Raes MB, Nef P (1995). "Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation". J. Recept. Signal Transduct. Res. 15 (1–4): 365–78. doi:10.3109/10799899509045227. PMID 8903951.
Further reading
- Dason JS, Romero-Pozuelo J, Atwood HL, Ferrús A (April 2012). "Multiple roles for frequenin/NCS-1 in synaptic function and development". Mol. Neurobiol. 45 (2): 388–402. doi:10.1007/s12035-012-8250-4. hdl:10261/60667. PMID 22396213. S2CID 12709387.
- Weiss JL, Hui H, Burgoyne RD (November 2010). "Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth". Cell. Mol. Neurobiol. 30 (8): 1283–92. doi:10.1007/s10571-010-9588-7. PMID 21104311. S2CID 2270302.