Olesoxime
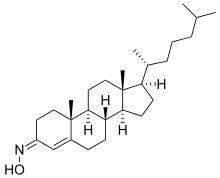
Olesoxime (TRO19622) is an experimental drug formerly under development by the now-defunct French company Trophos as a treatment for a range of neuromuscular disorders. It has a cholesterol-like structure and belongs to the cholesterol-oxime family of mitochondrial pore modulators.[1][2]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C27H45NO |
| Molar mass | 399.65 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Research
In preclinical studies, the compound displayed neuroprotective properties by promoting the function and survival of neurons and other cell types under disease-relevant stress conditions. It did so through interactions with the mitochondrial permeability transition pore (mPTP).[3]
A 2009–2011 phase 3 clinical trial in amyotrophic lateral sclerosis did not demonstrate an increase in survival.[4] A 2011–2013 trial in spinal muscular atrophy (SMA) indicated that the compound may prevent deterioration of muscle function.[5][6] In 2015, the entire olesoxime programme was purchased by Hoffmann-La Roche for €120 million with a view to developing a treatment for SMA. However, in June 2018, faced with technical and regulatory challenges and competition from a potentially more effective drug nusinersen, Roche halted further development of olesoxime.[7]
References
- Martin LJ (August 2010). "Olesoxime, a cholesterol-like neuroprotectant for the potential treatment of amyotrophic lateral sclerosis". IDrugs. 13 (8): 568–80. PMC 3058503. PMID 20721828.
- "Olesoxime". New Drugs Online Report. UK Medicines Information. Archived from the original on 2016-03-03.
- Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, Akentieva NP, Evers AS, Covey DF, Ostuni MA, Lacapère JJ, Massaad C, Schumacher M, Steidl EM, Maux D, Delaage M, Henderson CE, Pruss RM (August 2007). "Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis". The Journal of Pharmacology and Experimental Therapeutics. 322 (2): 709–20. doi:10.1124/jpet.107.123000. PMID 17496168. S2CID 17271734.
- "Trophos announces results of phase 3 study of olesoxime in Amyotrophic Lateral Sclerosis". Press Release. Trophos. 2011-12-13. Archived from the original on 2014-02-23.
- "Trophos announces top-line results of pivotal trial of olesoxime in spinal muscular atrophy". Press Release. Trophos. 2014-03-10. Archived from the original on 2014-12-11.
- Bertini E, Dessaud E, Mercuri E, Muntoni F, Kirschner J, Reid C, Lusakowska A, Comi GP, Cuisset JM, Abitbol JL, Scherrer B, Ducray PS, Buchbjerg J, Vianna E, van der Pol WL, Vuillerot C, Blaettler T, Fontoura P (July 2017). "Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: a randomised, double-blind, placebo-controlled phase 2 trial" (PDF). The Lancet. Neurology. 16 (7): 513–522. doi:10.1016/S1474-4422(17)30085-6. hdl:2434/501447. PMID 28460889. S2CID 5842023.
- Taylor, Nick P. (2018-06-01). "Roche scraps €120M SMA drug after hitting 'many difficulties'". www.fiercebiotech.com. Retrieved 2018-06-07.
Further reading
- Rovini A, Carré M, Bordet T, Pruss RM, Braguer D (September 2010). "Olesoxime prevents microtubule-targeting drug neurotoxicity: selective preservation of EB comets in differentiated neuronal cells". Biochemical Pharmacology. 80 (6): 884–94. doi:10.1016/j.bcp.2010.04.018. PMID 20417191.
- Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM (December 2009). "Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel". Pain. 147 (1–3): 202–9. doi:10.1016/j.pain.2009.09.006. PMC 2787910. PMID 19833436.
- Bordet T, Buisson B, Michaud M, Abitbol JL, Marchand F, Grist J, Andriambeloson E, Malcangio M, Pruss RM (August 2008). "Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy". The Journal of Pharmacology and Experimental Therapeutics. 326 (2): 623–32. doi:10.1124/jpet.108.139410. PMID 18492948. S2CID 33726393.
External links
- cholest-4-en-3-one, oxime at the US National Library of Medicine Medical Subject Headings (MeSH)'