Omecamtiv mecarbil
Omecamtiv mecarbil (INN), previously referred to as CK-1827452, is a cardiac-specific myosin activator. It is being studied for a potential role in the treatment of left ventricular systolic heart failure.[1]
 | |
| Clinical data | |
|---|---|
| Other names | CK-1827452 |
| Routes of administration | Intravenous infusion or oral tablet |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
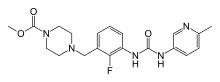
| Formula | C20H24FN5O3 |
| Molar mass | 401.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Systolic heart failure involves a loss of effective actin-myosin cross bridges in the myocytes (heart muscle cells) of the left ventricle, which leads to a decreased ability of the heart to move blood through the body. This causes peripheral edema (blood pooling), which the sympathetic nervous system tries to correct[2] by overstimulating the cardiac myocytes, leading to left ventricular hypertrophy, another characteristic of chronic heart failure.
Current inotropic therapies work by increasing the force of cardiac contraction, such as through calcium conduction or modulating adrenoreceptors. But these are limited by adverse events, including arrhythmias related to increased myocardical oxygen consumption, desensitization of adrenergic receptors, and altering intracellular calcium levels.[3] Inotropes are also thought to be associated with worse prognosis.[4] Therefore, the novel mechanism of omecamtiv mecarbil may offer a useful new option for heart failure.
Mechanism of action
Cardiac myocytes contract through a cross-bridge cycle between the myofilaments, actin and myosin. Chemical energy in the form of ATP is converted into mechanical energy which allows myosin to strongly bind to actin and produce a power stroke resulting in sarcomere shortening/contraction.[5] Omecamtiv mecarbil specifically targets and activates myocardial ATPase and improves energy utilization. This enhances effective myosin cross-bridge formation and duration, while the velocity of contraction remains the same.[6] Specifically, it increases the rate of phosphate release from myosin by stabilizing the pre-powerstroke and the phosphate release states,[7] thereby accelerating the rate-determining step of the cross-bridge cycle, which is the transition of the actin-myosin complex from the weakly bound to the strongly bound state.[8][1] Furthermore, once myosin is bound to actin, it stays bound dramatically longer in the presence of omecamtiv mecarbil.[9][10][7] The combination of increased and prolonged cross-bridge formation prolongs myocardial contraction. Thus, the overall clinical result of omecamtiv mecarbil is an increase in left ventricular systolic ejection time and ejection fraction.[6][8]
There is a slight decrease in heart rate while myocardial oxygen consumption is unaffected. The increased cardiac output is independent of intracellular calcium and cAMP levels.[3][11] Thus omecamtiv mecarbil improves systolic function by increasing the systolic ejection duration and stroke volume, without consuming more ATP energy, oxygen or altering intracellular calcium levels causing an overall improvement in cardiac efficiency.[6]
Clinical trials
Experimental studies on rats and dogs, proved the efficacy and mechanism of action of omecamtiv mecarbil.[3] Current clinical studies on humans have shown there is a direct linear relationship between dose and systolic ejection time.[1][12][13] The dose-dependent effects persisted throughout the entire trial, suggesting that desensitization does not occur. The maximum tolerated dose was observed to be an infusion of 0.5 mg/kg/h. Adverse effects, such as ischemia, were only seen at doses beyond this level, due to extreme lengthening of systolic ejection time.[1] Thus due to the unique cardiac myosin activation mechanism, omecamtiv mecarbil could safely improve cardiac function within tolerated doses. Omecamtiv mecarbil effectively relieves symptoms and enhances the quality of life of systolic heart failure patients. It drastically improves cardiac performance in the short term; however, the hopeful long-term effects of reduced mortality have yet to be studied.[1][2]
Myosin inhibition
Recently, research groups found that omecamtiv mecarbil actually inhibits myosin by enhancing the duty ratio, increasing calcium sensitivity and slowing force development.[14] It may still activate muscle as a whole however despite suppressing the working stroke of myosin.[15]
History
The U.S. Food and Drug Administration (FDA) granted in May 2020 a fast-track designation for omecamtiv mecarbil. The designation "represents an important milestone in the development of omecamtiv mecarbil," commented David Reese, head of R&D at Amgen, noting that "half of heart failure patients will die within five years of diagnosis, underscoring the urgent need for new therapies for this grievous condition".[16]
References
- Teerlink, JR (2009). "A novel approach to improve cardiac performance: cardiac myosin activators". Heart Fail Rev. 14 (4): 289–298. doi:10.1007/s10741-009-9135-0. ISSN 1382-4147. PMC 2772957. PMID 19234787.
- Dyke D, Koelling T (2008). "Heart failure due to left ventricular systolic dysfunction". In Eagle KA, Baliga RR (eds.). Practical Cardiology. Philadelphia: Lippincott Williams & Wilkins. pp. 246–285. ISBN 978-0-7817-7294-5.
- Shen YT, Malik FI, Zhao X, Depre C, Dhar SK, Abarzúa P, Morgans DJ, Vatner SF (Jul 2010). "Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure". Circ Heart Fail. 3 (4): 522–7. doi:10.1161/CIRCHEARTFAILURE.109.930321. PMID 20498236.
- Nieminen, M (March 2005). "Pharmacological options for acute heart failure syndromes: current treatments and unmet needs". Eur Heart J. 7: B20-4. doi:10.1093/eurheartj/sui009.
- Bers, DM (Jan 2002). "Cardiac excitation-contraction coupling". Nature. 415 (6868): 198–205. doi:10.1038/415198a. PMID 11805843.
- Malik F, Teerlink J, Escandon R, Clake C, Wolff A (2006). "The Selective Cardiac Myosin Activator, CK-1827452, a Calcium-Independent Inotrope, Increases Left Ventricular Systolic Function by Increasing Ejection Time Rather than the Velocity of Contraction". Circulation. 114 (18 Suppl): 441.CS1 maint: uses authors parameter (link)
- Planelles-Herrero, Vicente J.; Hartman, James J.; Robert-Paganin, Julien; Malik, Fady I.; Houdusse, Anne (2017). "Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil". Nature Communications. 8 (1): 190. doi:10.1038/s41467-017-00176-5. ISSN 2041-1723. PMC 5543065. PMID 28775348.
- Malik, Fady I.; Hartman, James J.; Elias, Kathleen A.; Morgan, Bradley P.; Rodriguez, Hector; Brejc, Katjuša; Anderson, Robert L.; Sueoka, Sandra H.; Lee, Kenneth H.; Finer, Jeffrey T.; Sakowicz, Roman; Baliga, Ramesh; Cox, David R.; Garard, Marc; Godinez, Guillermo; Kawas, Raja; Kraynack, Erica; Lenzi, David; Lu, Pu Ping; Muci, Alexander; Niu, Congrong; Qian, Xiangping; Pierce, Daniel W.; Pokrovskii, Maria; Suehiro, Ion; Sylvester, Sheila; Tochimoto, Todd; Valdez, Corey; Wang, Wenyue; Katori, Tatsuo; Kass, David A.; Shen, You-Tang; Vatner, Stephen F.; Morgans, David J. (18 March 2011). "Cardiac Myosin Activation: A Potential Therapeutic Approach for Systolic Heart Failure". Science. 331 (6023): 1439–1443. doi:10.1126/science.1200113. ISSN 0036-8075. PMC 4090309. PMID 21415352.
- Liu, Chao; Kawana, Masataka; Song, Dan; Ruppel, Kathleen M.; Spudich, James A. (June 2018). "Controlling load-dependent kinetics of β-cardiac myosin at the single-molecule level". Nature Structural & Molecular Biology. 25 (6): 505–514. doi:10.1038/s41594-018-0069-x. ISSN 1545-9993. PMC 6092189. PMID 29867217.
- Woody, Michael S.; Greenberg, Michael J.; Barua, Bipasha; Winkelmann, Donald A.; Goldman, Yale E.; Ostap, E. Michael (11 April 2018). "Positive Cardiac Inotrope, Omecamtiv Mecarbil, Activates Muscle Despite Suppressing the Myosin Working Stroke". bioRxiv: 298141. doi:10.1101/298141.
- Teerlink JR, Metra M, Zacà V, Sabbah HN, Cotter G, Gheorghiade M, Cas LD (Dec 2009). "Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond". Heart Fail Rev. 14 (4): 243–53. doi:10.1007/s10741-009-9153-y. PMC 2772951. PMID 19876734.
- Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA (Aug 2011). "Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study". Lancet. 378 (9792): 667–75. doi:10.1016/S0140-6736(11)61219-1. PMID 21856480.
- Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI (Aug 2011). "The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial". Lancet. 378 (9792): 676–83. doi:10.1016/S0140-6736(11)61126-4. PMID 21856481.
- Swenson AM, et al. Omecamtiv mecarbil enhances the duty ratio of human β-cardiac myosin resulting in increased calcium sensitivity and slowed force development in cardiac muscle. J Biol Chem. 2017;292:3768–3778
- Woody MS, et al. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun. 2018;9:3838.
- "FDA Grants Fast Track Designation For Omecamtiv Mecarbil In Heart Failure" (Press release). AMGEN. May 8, 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.