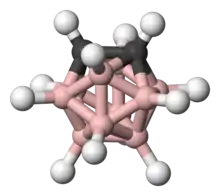
Ortho-carborane
Ortho-carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C.
 | |
| Names | |
|---|---|
| Other names
1,2-Dicarbadodecaborane(12), ortho-dicarbadodecaborane | |
| Identifiers | |
| EC Number |
|
| Properties | |
| C2H12B10 | |
| Molar mass | 144.22 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 320 °C (608 °F; 593 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H228, H302, H312, H332 | |
| P210, P240, P241, P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P370+378, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
The preparation of closo-dicarbadodecaboranes was reported independently by groups at Olin Corporation and the Reaction Motors Division of Thiokol Chemical Corporation working under the U.S. Air Force and published in 1963. These groups demonstrated the high stability in air of 1,2-closo-dodecaborane and related compounds, presented a general synthesis, described the transformation of substituents without destroying the carborane cluster, and demonstrated the ortho to meta isomerization.[1] The cluster has C2v symmetry.[2]
Ortho-carborane is prepared by the addition of acetylenes to decaborane(14). Modern syntheses involve two stages, the first involving generation of an adduct of decaborane:[3][4]
- B10H14 + 2 SEt2 → B10H12(SEt2)2 + H2
In the second stage, the alkyne is installed as the source of two carbon vertices:[4]
- B10H12(SEt2)2 + C2H2 → C2B10H12 + 2 SEt2 + H2
In place of acetylene itself, protected versions of C2H2 can be employed more conveniently than acetylene gas:
- B10H12(SEt2)2 + C2(CH2O2CCH3)2 → C2B10H10(CH2O2CCH3)2 + 2 SEt2 + H2
The organic substituents are removed by oxidation and hydrolysis:[3]
- 3 C2B10H10(CH2O2CH3)2 + 10 KOH + + 8 KMnO4 → 3 C2B10H12 + 6 CH3CO2K + 8 MnO2 + 6 K2CO3 + 8 H2O
Reactions
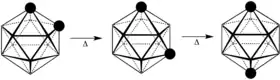
Upon heating to 420 °C, it rearranges to form the meta isomer. The para isomer is produced by heating to temperatures above 600 °C.
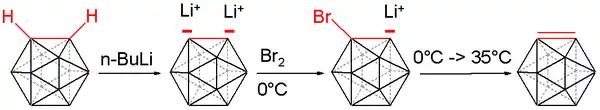
Treatment with organolithium reagents gives the dilithio derivative.[5]
- C2B10H12 + 2 BuLi → Li2C2B10H10 + 2 BuH
This dilithiated compound reacts with a variety of electrophiles, e.g. chlorophosphines, chlorosilanes, and sulfur.[6]
Base degradation of ortho carborane gives the anionic 11-vertex derivative, precursor to dicarbollide complexes:[7]
- C2B10H12 + NaOEt + 2 EtOH → Na+C2B9H12− + H2 + B(OEt)3
The use of dicarbollides (C2B8H112-) as ligands was developed by M. Frederick Hawthorne and co-workers.[8] The dianion forms sandwich compounds, bis(dicarbollides). Dicarbollides, being strong electron donors, stabilize higher oxidation states, e.g. Ni(IV).
ortho-Carborane can be converted to highly reactive carborynes with the formula B10C2H10.
References
- Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. (1963). "A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds". Inorganic Chemistry. 2 (6): 1089–1092. doi:10.1021/ic50010a002.
- Davidson, M. G.; Hibbert, T. G.; Howard, J. A. K.; Mackinnon, A.; Wade, K. (1996). "Definitive crystal structures of ortho-, meta- and para-carboranes: supramolecular structures directed solely by C–H⋯O hydrogen bonding to hmpa (hmpa = hexamethylphosphoramide)". Chem. Commun.: 2285–2286. doi:10.1039/CC9960002285.CS1 maint: uses authors parameter (link)
- Charles R. Kutal David A. Owen Lee J. Todd (1968). "closo‐1,2‐Dicarbadodecaborane(12)". Inorganic Syntheses. 11: 19–24. doi:10.1002/9780470132425.ch5.CS1 maint: uses authors parameter (link)
- M. Frederick Hawthorne, Timothy D. Andrews, Philip M. Garrett, Fred P. Olsen, Marten Reintjes, Fred N. Tebbe, Les F. Warren, Patrick A. Wegner, Donald C. Young (1967). "Icosahedral Carboranes and Intermediates Leading to the Preparation of Carbametallic Boron Hydride Derivatives". Inorganic Syntheses. 10: 91–118. doi:10.1002/9780470132418.ch17.CS1 maint: uses authors parameter (link)
- Popescu, A.-R.; Musteti, A. D.; Ferrer-Ugalde, A.; Viñas, C.; Núñez, R.; Teixidor, F. (2012). "Influential Role of Ethereal Solvent on Organolithium Compounds: The Case of Carboranyllithium". Chemistry – A European Journal. 18: 3174–3184. doi:10.1002/chem.201102626.CS1 maint: uses authors parameter (link)
- Jin, G.-X. (2004). "Advances in the Chemistry of Organometallic Complexes with 1,2-Dichalcogenolato-o-Carborane Ligands". Coord. Chem. Rev. 248: 587–602. doi:10.1016/j.ccr.2004.01.002.
- Plešek, J.; Heřmánek, S.; Štíbr, B. (1983). "Potassium dodecahydro-7,8-dicarba-nido-undecaborate(1-), k[7,8-C2B9H12], intermediates, stock solution, and anhydrous salt". Inorganic Syntheses. 22: 231–234. doi:10.1002/9780470132531.ch53.
- Hawthorne, M. F.; Young, D. C.; Wegner, P. A. (1965). "Carbametallic Boron Hydride Derivatives. I. Apparent Analogs of Ferrocene and Ferricinium Ion". Journal of the American Chemical Society. 87 (8): 1818–1819. doi:10.1021/ja01086a053.