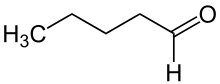
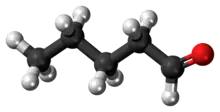
Pentanal
Pentanal (also called valeraldehyde) is the organic compound is an alkyl aldehyde, molecular formula C5H10O. It is used in flavorings, resin chemistry, and rubber accelerators.[1] Its smell is described as fermented, bready, fruity, nutty, berry.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Pentanal | |
| Other names
Pentanaldehyde Valeraldehyde Valeric aldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.442 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.134 g·mol−1 |
| Appearance | Clear liquid |
| Odor | Strong, acrid, pungent |
| Density | 0.8095 at 20 °C |
| Melting point | −60 °C (−76 °F; 213 K) |
| Boiling point | 102 to 103 °C (216 to 217 °F; 375 to 376 K) |
| Very slightly soluble | |
| Vapor pressure | 26 mmHg (20° C)[3] |
| Hazards | |
| Flash point | 12 °C; 54 °F; 285 K [3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[3] |
REL (Recommended) |
TWA 50 ppm (175 mg/m3)[3] |
IDLH (Immediate danger) |
N.D.[3] |
| Related compounds | |
Related aldehydes |
Butyraldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Pentanal is obtained by hydroformylation of butene. Also C4 mixtures can be used as starting material like the so-called raffinate II, which is produced by steam cracking and contains (Z)- and (E)-2-butene, 1-butene, butane and isobutane. The conversion to the product is accomplished with synthesis gas in the presence of a catalyst consisting of a rhodium-bisphosphite complex and a sterically hindered secondary amine with a selectivity toward pentanal of at least 90%.[5]
Use
Pentanal is used in diverse flavors (e. g. fruit flavors) and as a vulcanization accelerator.
2-Propyl-2-heptenal is obtained from pentanal by aldol condensation, which is hydrogenated to the saturated branched 2-propylheptanol. This alcohol serves as a starting material for the PVC plasticizer di-2-propylheptyl phthalate (DPHP).
Valeraldehyde is oxidized to give valeric acid.[6]
References
- Merck Index, 11th Edition, 9813.
- n-Valeraldehyde at chemicalland21.com
- NIOSH Pocket Guide to Chemical Hazards. "#0652". National Institute for Occupational Safety and Health (NIOSH).
- http://www.thegoodscentscompany.com/data/rw1009331.html
- Patent WO 2009/146985 der Evonik Oxeno GmbH.
- Riemenschneider, Wilhelm (2002). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235.
