Peroxide
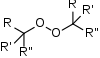
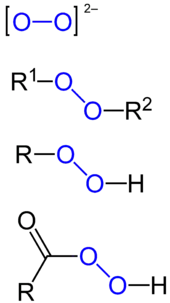
Peroxides are a group of compounds with the structure R−O−O−R.[1] The O−O group in a peroxide is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation number of −1.
 | |
| Names | |
|---|---|
| Other names
# peroxide | |
| Identifiers | |
| |
| Properties | |
| R−O−O−R | |
| Molar mass | 31.998 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
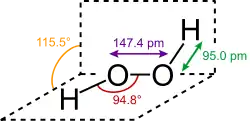
The most common peroxide is hydrogen peroxide (H2O2), colloquially known simply as "peroxide". It is marketed as a solution in water at various concentrations. Since hydrogen peroxide is nearly colorless, so are these solutions. It is mainly used as an oxidant and bleaching agent. However, hydrogen peroxide is also biochemically produced in the human body, largely as a result of a range of oxidase enzymes.[2] Concentrated solutions are potentially dangerous when in contact with organic compounds.
Aside from hydrogen peroxide, some other major classes of peroxides are:
- Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate.
- Main group peroxides, compounds with the linkage E−O−O−E (E = main group element).
- Metal peroxides, examples being barium peroxide (BaO2), sodium peroxide (Na2O2) and zinc peroxide (ZnO2).
- Organic peroxides, compounds with the linkage C−O−O−C or C−O−O−H. One example is tert-butylhydroperoxide.
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "peroxides". doi:10.1351/goldbook.P04510
- Halliwell, Barry; Clement, Marie Veronique; Long, Lee Hua (2000). "Hydrogen peroxide in the human body". FEBS Letters. 486 (1): 10–3. doi:10.1016/S0014-5793(00)02197-9. PMID 11108833.