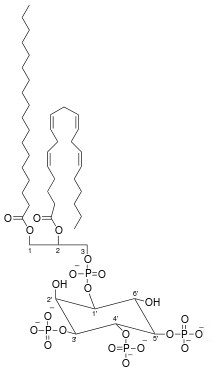
Phosphatidylinositol (3,4,5)-trisphosphate
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
 | |
| Names | |
|---|---|
| Other names
PI(3,4,5)P3, PtdIns(3,4,5)P3 | |
| Identifiers | |
| Properties | |
| C47H86O22P4 | |
| Molar mass | 1126.46 g/mol, neutral with fatty acid composition - 18:0, 20:4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Discovery
In 1988, Lewis C. Cantley published a paper describing the discovery of a novel type of phosphoinositide kinase with the unprecedented ability to phosphorylate the 3' position of the inositol ring resulting in the formation of phosphatidylinositol-3-phosphate (PI3P).[1] Working independently, Alexis Traynor-Kaplan and coworkers published a paper demonstrating that a novel lipid, phosphatidylinositol 3,4,5 trisphosphate (PIP3) occurs naturally in human neutrophils with levels that increased rapidly following physiologic stimulation with chemotactic peptide.[2] Subsequent studies demonstrated that in vivo the enzyme originally identified by Cantley's group prefers PtdIns(4,5)P2 as a substrate, producing the product PIP3.[3]
Function
PIP3 functions to activate downstream signaling components, the most notable one being the protein kinase AKT, which activates downstream anabolic signaling pathways required for cell growth and survival.[4]
PtdIns(3,4,5)P3 is dephosphorylated by the phosphatase PTEN on the 3 position, generating PI(4,5)P2, and by SHIPs (SH2-containing inositol phosphatase) on the 5' position of the inositol ring, producing PI(3,4)P2.[5]
The PH domain in a number of proteins binds to PtdIns(3,4,5)P3. Such proteins include Akt/PKB,[6] PDK1,[7] Btk1, and ARNO.[8]
Notable roles in the nervous system
PIP3 continues to play a critical role outside of the cytosol, notably at the postsynaptic terminal of hippocampal cells. Here, PIP3 has been implicated in regulating synaptic strengthening and AMPA expression, contributing to long-term potentiation. Moreover, PIP3 suppression disrupts normal AMPA expression on the neuron membrane and instead leads to the accumulation of AMPA on dendritic spines, commonly associated with synaptic depression.[9]
Although clearly an important molecule alone, it is notable that PIP3 interacts with other proteins to mediate synaptic plasticity. Of these proteins, Phldb2 has been shown to interact with PIP3 to induce and maintain LTP. In the absence of such an interaction, memory consolidation is impaired.[10]
References
- Whitman M, Downes CP, Keeler M, Keller T, Cantley L (April 1988). "Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate". Nature. 332 (6165): 644–6. Bibcode:1988Natur.332..644W. doi:10.1038/332644a0. PMID 2833705. S2CID 4326568.
- Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA (July 1988). "An inositol tetrakisphosphate-containing phospholipid in activated neutrophils". Nature. 334 (6180): 353–6. Bibcode:1988Natur.334..353T. doi:10.1038/334353a0. PMID 3393226. S2CID 4263472.
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC (April 1989). "PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells". Cell. 57 (1): 167–75. doi:10.1016/0092-8674(89)90182-7. PMID 2467744. S2CID 22154860.
- Ma, Qi; Zhu, Chongzhuo; Zhang, Weilin; Ta, Na; Zhang, Rong; Liu, Lei; Feng, Du; Cheng, Heping; Liu, Junling; Chen, Quan (January 2019). "Mitochondrial PIP3-binding protein FUNDC2 supports platelet survival via AKT signaling pathway". Cell Death and Differentiation. 26 (2): 321–331. doi:10.1038/s41418-018-0121-8. ISSN 1476-5403. PMC 6329745. PMID 29786068.
- Qi, Yanmei; Liu, Jie; Chao, Joshua; Greer, Peter A.; Li, Shaohua (2020-09-07). "PTEN dephosphorylates Abi1 to promote epithelial morphogenesis". The Journal of Cell Biology. 219 (9). doi:10.1083/jcb.201910041. ISSN 1540-8140. PMC 7480098. PMID 32673396.
- Eramo, Matthew J.; Mitchell, Christina A. (February 2016). "Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases". Biochemical Society Transactions. 44 (1): 240–252. doi:10.1042/BST20150214. ISSN 1470-8752. PMID 26862211.
- Gagliardi, Paolo Armando; Puliafito, Alberto; Primo, Luca (February 2018). "PDK1: At the crossroad of cancer signaling pathways". Seminars in Cancer Biology. 48: 27–35. doi:10.1016/j.semcancer.2017.04.014. ISSN 1096-3650. PMID 28473254.
- Venkateswarlu, Kanamarlapudi; Oatey, Paru B.; Tavaré, Jeremy M.; Cullen, Peter J. (April 1998). "Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase". Current Biology. 8 (8): 463–466. doi:10.1016/s0960-9822(98)70181-2. ISSN 0960-9822. PMID 9550703. S2CID 12974067.
- Arendt, Kristin L.; Royo, María; Fernández-Monreal, Mónica; Knafo, Shira; Petrok, Cortney N.; Martens, Jeffrey R.; Esteban, José A. (January 2010). "PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane". Nature Neuroscience. 13 (1): 36–44. doi:10.1038/nn.2462. ISSN 1546-1726. PMC 2810846. PMID 20010819.
- Xie, Min-Jue; Ishikawa, Yasuyuki; Yagi, Hideshi; Iguchi, Tokuichi; Oka, Yuichiro; Kuroda, Kazuki; Iwata, Keiko; Kiyonari, Hiroshi; Matsuda, Shinji; Matsuzaki, Hideo; Yuzaki, Michisuke (13 March 2019). "PIP3-Phldb2 is crucial for LTP regulating synaptic NMDA and AMPA receptor density and PSD95 turnover". Scientific Reports. 9 (1): 4305. Bibcode:2019NatSR...9.4305X. doi:10.1038/s41598-019-40838-6. ISSN 2045-2322. PMC 6416313. PMID 30867511.