Plant embryogenesis
Plant embryogenesis is a process that occurs after the fertilization of an ovule to produce a fully developed plant embryo. This is a pertinent stage in the plant life cycle that is followed by dormancy and germination.[1] The zygote produced after fertilization must undergo various cellular divisions and differentiations to become a mature embryo.[1] An end stage embryo has five major components including the shoot apical meristem, hypocotyl, root meristem, root cap, and cotyledons.[1] Unlike animal embryogenesis, plant embryogenesis results in an immature form of the plant, lacking most structures like leaves, stems, and reproductive structures.[2] However, both plants and animals pass through a phylotypic stage that evolved independently[3] and that causes a developmental constraint limiting morphological diversification.[4][5][6][7]
Morphogenic events
Embryogenesis occurs naturally as a result of single, or double fertilization, of the ovule, giving rise to two distinct structures: the plant embryo and the endosperm which go on to develop into a seed.[8] The zygote goes through various cellular differentiations and divisions in order to produce a mature embryo. These morphogenic events form the basic cellular pattern for the development of the shoot-root body and the primary tissue layers; it also programs the regions of meristematic tissue formation. The following morphogenic events are only particular to eudicots, and not monocots.
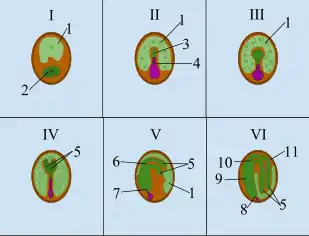
|
|
Two cell stage
Following fertilization, the zygote and endosperm are present within the ovule, as seen in stage I of the illustration on this page. Then the zygote undergoes an asymmetric transverse cell division that gives rise to two cells - a small apical cell resting above a large basal cell.[9][10] These two cells are very different, and give rise to different structures, establishing polarity in the embryo.
- apical cell
- The small apical cell is on the top and contains most of the cytoplasm, the aqueous substance found within cells, from the original zygote.[11] It gives rise to the hypocotyl, shoot apical meristem, and cotyledons.[11]
- basal cell
- The large basal cell is on the bottom and consists of a large vacuole[11] and gives rise to the hypophysis[9] and the suspensor.[9]
Eight cell stage
After two rounds of longitudinal division, and one round of transverse division, an eight-celled embryo is the result.[12] Stage II, in the illustration above, indicates what the embryo looks like during the eight cell stage. According to Laux et al., there are four distinct domains during the eight cell stage.[13] The first two domains contribute to the embryo proper. The apical embryo domain, gives rise to the shoot apical meristem and cotyledons. The second domain, the central embryo domain, gives rise to the hypocotyl, root apical meristem, and parts of the cotyledons. The third domain, the basal embryo domain, contains the hypophysis. The hypophysis will later give rise to the radicle and the root cap. The last domain, the suspensor, is the region at the very bottom, which connects the embryo to the endosperm for nutritional purposes.
Sixteen cell stage
Additional cell divisions occur, which leads to the sixteen cell stage. The four domains are still present, but they are more defined with the presence of more cells. The important aspect of this stage is the introduction of the protoderm, which is meristematic tissue that will give rise to the epidermis.[12] The protoderm is the outermost layer of cells in the embryo proper.[12]
Globular stage
The name of this stage is indicative of the embryo's appearance at this point in embryogenesis; it is spherical or globular. Stage III, in the photograph above, depicts what the embryo looks like during the globular stage. 1 is indicating the location of the endosperm. The important component of the globular phase is the introduction of the rest of the primary meristematic tissue. The protoderm was already introduced during the sixteen cell stage. According to Evert and Eichhorn, the ground meristem and procambium are initiated during the globular stage.[12] The ground meristem will go on to form the ground tissue, which includes the pith and cortex. The procambium will eventually form the vascular tissue, which includes the xylem and phloem.
Heart stage
According to Evert and Eichhorn, the heart stage is a transition period where the cotyledons finally start to form and elongate.[12] It is given this name in eudicots because most plants from this group have two cotyledons, giving the embryo a heart shaped appearance. The shoot apical meristem is between the cotyledons. Stage IV, in the illustration above, indicates what the embryo looks like at this point in development. 5 indicates the position of the cotyledons.
Pro embryo stage
This stage is defined by the continued growth of the cotyledons and axis elongation.[12] In addition, programmed cell death must occur during this stage. This is carried out throughout the entire growth process, like any other development.[14] However, in the torpedo stage of development, parts of the suspensor complex must be terminated.[14] The suspensor complex is shortened because at this point in development most of the nutrition from the endosperm has been utilized, and there must be space for the mature embryo.[11] After the suspensor complex is gone, the embryo is fully developed.[13] Stage V, in the illustration above, indicates what the embryo looks like at this point in development.
Maturation
The second phase, or postembryonic development, involves the maturation of cells, which involves cell growth and the storage of macromolecules (such as oils, starches and proteins) required as a 'food and energy supply' during germination and seedling growth. The appearance of a mature embryo is seen in Stage VI, in the illustration above.
Dormancy
The end of embryogenesis is defined by an arrested development phase, or stop in growth. This phase usually coincides with a necessary component of growth called dormancy. Dormancy is a period in which a seed cannot germinate, even under optimal environmental conditions, until a specific requirement is met.[15] Breaking dormancy, or finding the specific requirement of the seed, can be rather difficult. For example, a seed coat can be extremely thick. According to Evert and Eichhorn, very thick seed coats must undergo a process called scarification, in order to deteriorate the coating.[12] In other cases, seeds must experience stratification. This process exposes the seed to certain environmental conditions, like cold or smoke, to break dormancy and initiate germination.
The role of auxin
Auxin is a hormone related to the elongation and regulation of plants.[16] It also plays an important role in the establishment polarity with the plant embryo. Research has shown that the hypocotyl from both gymnosperms and angiosperms show auxin transport to the root end of the embryo.[17] They hypothesized that the embryonic pattern is regulated by the auxin transport mechanism and the polar positioning of cells within the ovule. The importance of auxin was shown, in their research, when carrot embryos, at different stages, were subjected to auxin transport inhibitors. The inhibitors that these carrots were subjected to made them unable to progress to later stages of embryogenesis. During the globular stage of embryogenesis, the embryos continued spherical expansion. In addition, oblong embryos continued axial growth, without the introduction of cotyledons. During the heart embryo stage of development, there were additional growth axes on hypocotyls. Further auxin transport inhibition research, conducted on Brassica juncea, shows that after germination, the cotyledons were fused and not two separate structures.[18]
Alternative forms of embryogenesis
Somatic embryogenesis
Somatic embryos are formed from plant cells that are not normally involved in the development of embryos, i.e. ordinary plant tissue. No endosperm or seed coat is formed around a somatic embryo. Applications of this process include: clonal propagation of genetically uniform plant material; elimination of viruses; provision of source tissue for genetic transformation; generation of whole plants from single cells called protoplasts; development of synthetic seed technology. Cells derived from competent source tissue are cultured to form an undifferentiated mass of cells called a callus. Plant growth regulators in the tissue culture medium can be manipulated to induce callus formation and subsequently changed to induce embryos to form the callus. The ratio of different plant growth regulators required to induce callus or embryo formation varies with the type of plant. Asymmetrical cell division also seems to be important in the development of somatic embryos, and while failure to form the suspensor cell is lethal to zygotic embryos, it is not lethal for somatic embryos.
Androgenesis
The process of androgenesis allows a mature plant embryo to form from a reduced, or immature, pollen grain.[19] Androgenesis usually occurs under stressful conditions.[19] Embryos that result from this mechanism can germinate into fully functional plants. As mentioned, the embryo results from a single pollen grain. Pollen grains consists of three cells - one vegetative cell containg two generative cells. According to Maraschin et al., androgenesis must be triggered during the asymmetric division of microspores.[19] However, once the vegetative cell starts to make starch and proteins, androgenesis can no longer occur. Maraschin et al., indicates that this mode of embryogenesis consists of three phases. The first phase is the acquisition of embryonic potential, which is the repression of gametophyte formation, so that the differentiation of cells can occur. Then during the initiation of cell divisions, multicellular structures begin to form, which are contained by the exine wall. The last step of androgenesis is pattern formation, where the embryo-like structures are released out of the exile wall, in order for pattern formation to continue.
After these three phases occur, the rest of the process falls in line with the standard embryogenesis events.
Plant growth and buds
Embryonic tissue is made up of actively growing cells and the term is normally used to describe the early formation of tissue in the first stages of growth. It can refer to different stages of the sporophyte and gametophyte plant; including the growth of embryos in seedlings, and to meristematic tissues,[20] which are in a persistently embryonic state,[21] to the growth of new buds on stems.[22]
In both gymnosperms and angiosperms, the young plant contained in the seed, begins as a developing egg-cell formed after fertilization (sometimes without fertilization in a process called apomixis) and becomes a plant embryo. This embryonic condition also occurs in the buds that form on stems. The buds have tissue that has differentiated but not grown into complete structures. They can be in a resting state, lying dormant over winter or when conditions are dry, and then commence growth when conditions become suitable. Before they start growing into stem, leaves, or flowers, the buds are said to be in an embryonic state.
Notes and references
- Goldberg, Robert; Paiva, Genaro; Yadegari, Ramin (October 28, 1994). "Plant Embryogenesis: Zygote to Seed". Science. 266 (5185): 605–614. Bibcode:1994Sci...266..605G. doi:10.1126/science.266.5185.605. PMID 17793455. S2CID 5959508.
- Jurgens, Gerd (May 19, 1995). "Axis formation in plant embryogenesis: cues and clues". Cell. 81 (4): 467–470. doi:10.1016/0092-8674(95)90065-9. PMID 7758100. S2CID 17143479.
- Drost, Hajk-Georg; Janitza, Philipp; Grosse, Ivo; Quint, Marcel (2017). "Cross-kingdom comparison of the developmental hourglass". Current Opinion in Genetics & Development. 45: 69–75. doi:10.1016/j.gde.2017.03.003. PMID 28347942.
- Irie, Naoki; Kuratani, Shigeru (2011-03-22). "Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis". Nature Communications. 2: 248. doi:10.1038/ncomms1248. ISSN 2041-1723. PMC 3109953. PMID 21427719.
- Domazet-Lošo, Tomislav; Tautz, Diethard (2010-12-09). "A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns". Nature. 468 (7325): 815–818. doi:10.1038/nature09632. ISSN 0028-0836. PMID 21150997. S2CID 1417664.
- Quint, Marcel; Drost, Hajk-Georg; Gabel, Alexander; Ullrich, Kristian Karsten; Bönn, Markus; Grosse, Ivo (2012-10-04). "A transcriptomic hourglass in plant embryogenesis". Nature. 490 (7418): 98–101. doi:10.1038/nature11394. ISSN 0028-0836. PMID 22951968. S2CID 4404460.
- Drost, Hajk-Georg; Gabel, Alexander; Grosse, Ivo; Quint, Marcel (2015-05-01). "Evidence for Active Maintenance of Phylotranscriptomic Hourglass Patterns in Animal and Plant Embryogenesis". Molecular Biology and Evolution. 32 (5): 1221–1231. doi:10.1093/molbev/msv012. ISSN 0737-4038. PMC 4408408. PMID 25631928.
- Radoeva, Tatyana; Weijers, Dolf (November 2014). "A roadmap to embryo identity in plants". Trends in Plant Science. 19 (11): 709–716. doi:10.1016/j.tplants.2014.06.009. PMID 25017700.
- West, Marilyn A. L.; Harada, John J. (October 1993). "Embryogenesis in Higher Plants: An Overview". The Plant Cell. 5 (10): 1361–1369. doi:10.2307/3869788. JSTOR 3869788. PMC 160368. PMID 12271035.
- Peris, Cristina I. Llavanta; Rademacher, Eike H.; Weijers, Dolf (2010). "Chapter 1 Green Beginnings - Pattern Formation in the Early Plant Embryo". In Timmermans, Marja C. P. (ed.). Plant development (1st ed.). San Diego, CA: Academic Press (imprint of Elsevier). pp. 1–27. ISBN 978-0-12-380910-0.
- Souter, Martin; Lindsey, Keith (June 2000). "Polarity and signaling in plant embryogenesis". Journal of Experimental Botany. 51 (347): 971–983. doi:10.1093/jexbot/51.347.971. PMID 10948225.
- Evert, Ray F.; Eichhorn, Susan E. (2013). Raven Biology of Plants. New York: W. H. Freeman and Company. pp. 526–530.
- Laux, T.; Wurschum, T.; Breuninger, Holger (2004). "Genetic Regulation of Embryonic Pattern Formation". The Plant Cell. 6: 190–202. doi:10.1105/tpc.016014. PMC 2643395. PMID 15100395.
- Bozhkov, P. V.; Filonova, L. H.; Suarez, M. F. (January 2005). "Programmed cell death in plant embryogenesis". Current Topics in Developmental Biology. 67: 135–179. doi:10.1016/S0070-2153(05)67004-4. ISBN 9780121531676. PMID 15949533.
- Baskin, Jeremy M.; Baskin, Carol C. (2004). "A classification system for seed dormancy" (PDF). Seed Science Research. 14: 1–16. doi:10.1079/SSR2003150 – via Google Scholar.
- Liu, C; Xu, Z; Chua, N (1993). "Auxin Polar Transport Is Essential for the Establishment of Bilateral Symmetry during Early Plant Embryogenesis". The Plant Cell. 5 (6): 621–630. doi:10.2307/3869805. JSTOR 3869805. PMC 160300. PMID 12271078.
- Cooke, T. J.; Racusen, R. H.; Cohen, J. D. (November 1993). "The role of auxin in plant embryogenesis". Plant Cell. 11 (11): 1494–1495. doi:10.1105/tpc.5.11.1494. PMC 160380. PMID 12271044.
- Hadfi, K.; Speth, V.; Neuhaus, G. (1998). "Auxin-induced developmental patterns in Brassica juncea embryos". Development. 125 (5): 879–87. PMID 9449670.
- Maraschin, S. F.; de Priester, W.; Spaink, H. P.; Wang, M. (July 2005). "Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective". Journal of Experimental Botany. 56 (417): 1711–1726. doi:10.1093/jxb/eri190. PMID 15928015.
- Pandey, Brahma Prakash. 2005. Textbook of botany angiosperms: taxonomy, anatomy, embryology (including tissue culture) and economic botany. New Delhi: S. Chand & Company. p 410.
- McManus, Michael T., and Bruce E. Veit. 2002. Meristematic tissues in plant growth and development. Sheffield: Sheffield Academic Press.
- Singh, Gurcharan. 2004. Plant systematics: an integrated approach. Enfield, NH: Science Publishers. p 61.
External links
| Look up hypophysis in Wiktionary, the free dictionary. |
