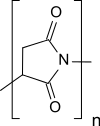
Polysuccinimide
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.
 | |
| Identifiers | |
|---|---|
| Properties | |
| (C4H3NO2)n | |
| Molar mass | 97.07 g·mole−1 |
| Appearance | solid |
| * insoluble in water[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name is derived from the salt of succinic acid, the structurally related succinate.
Production
The production of polysuccinimide was reported by Hugo Schiff as early as 1897.[5] When dry aspartic acid was heated for about 20 hours at 190 °C to 200 °C, a colorless product was obtained. Above 200 °C, a weak yellowing occurs, the yield was almost quantitative.[6]
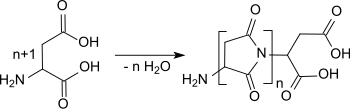
In the experiments by Hugo Schiff, oligomers and low-molecular polymers were formed in a solid state reaction by polycondensation upon water elimination. This is generally the case in the absence of strong acids, which suppress the thermal decomposition of free amino end groups and thus chain interruption reactions. The formation of the polyimide polysuccinimide can be followed by the intensive absorption band in the infrared spectrum at 1714 cm−1. Many process variants described in the patent literature yield besides a relatively low degree of polymerization often branched and yellow to brown discolored products.[7]
Recent work has focused on increasing the molar mass and achieving a linear chain structure while avoiding decomposition reactions. With a simple "oven process" in which a mixture or paste of crystalline aspartic acid and concentrated phosphoric acid or polyphosphoric acid in a thin layer is heated to 200 °C for 2 to 4 hours, polysuccinimide is produced with molar masses in the range of 30,000 g/mol and cream white shade.[8] The implementation of the polycondensation in several steps[9] (precondensation, comminution, postcondensation), with other dehydrating substances (for example zeolites, triphenyl phosphite[10]) or in the presence of solvents[11] (for example propylene carbonate) provides higher molecular weight products with molar masses in the range of 10,000 to 200,000 g/mol. However, the patent literature does not address the polymer morphology, in particular the degree of branching.
A recent patent[12] describes the simple preparation of high molecular weight, virtually colorless and linear, unbranched polysuccinimide. For this purpose, aspartic acid, which is present as crystalline zwitterion and practically water-insoluble, is firstly dissolved with an aqueous, volatile acid (preferably hydrochloric acid) and mixed with phosphoric acid as condensing agent. The resulting homogeneous solution is evaporated at 120 °C and the resulting glassy mass is then polycondensed at 180 °C to 200 °C for at least one hour. The phosphoric acid is washed out and the dried polysuccinimide is converted by mild alkaline hydrolysis into water-soluble polyaspartic acid; the molar mass of which can be determined by gel permeation chromatography. The process provides reproducible polysuccinimide with molar masses above 100,000 g/mol.
Synthetic routes for polysuccinimides based on maleic acid monoammonium salt,[13] maleic anhydride and ammonia[14] or based on the intermediately formed maleic acid monoamide[15] achieved only low molar masses of a few 1,000 g/mol and yielded colored products. The same was the case for "green" process variants in supercritical carbon dioxide and while avoiding mineral acids as catalysts.[6]
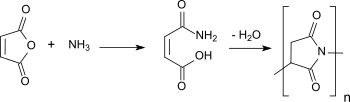
Due to the lower cost of maleic anhydride and ammonia, starting materials produced from fossil raw materials, no L-aspartic acid (of biogenic origin) is used in the production of the commercial product Baypure® polysuccinimide either.
Properties
Polysuccinimide is produced as an odourless, non-hygroscopic, cream-white to brown powder which is soluble in aprotic dipolar solvents such as dimethylformamide, dimethylacetamide, dimethylsulfoxide, N-methylpyrrolidone, triethylene glycol or mesitylene/sulfolane mixtures. Polysuccinimide hydrolyses in water only very slowly. In diluted alkaline media (e.g. 1M sodium hydroxide solution), hydrolysis takes place in α- and β-position of the succinimide (2,5-pyrrolidinedione) ring structures and racemization follows at the chiral center of the aspartic acid, yielding the water-soluble sodium salt of the poly(α, β)-DL-aspartic acid. The α form is formed to approx. 30 %, the β form to approx. 70 % in random arrangement along the polymer chain.[16]
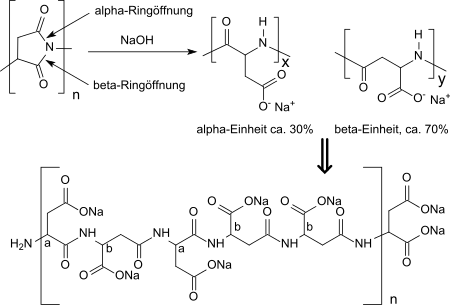
In more basic solutions or with longer reaction times, the amide linkages in the polymer chain are attacked upon degradation of the molar mass. The presence of amide bonds makes the polyaspartic acid obtained in the hydrolysis relatively biodegradable (about 70% in wastewater), even of initially highly crosslinked polysuccinimides.[17]
Use
The polysuccinimide[4] developed[18] by Bayer AG and marketed by Lanxess AG under the brand name Baypure® DSP with an average molecular weight of 4,400 g/mol is partially hydrolyzed even at slightly elevated pH values and is thus swellable in highly crosslinked form or water-soluble in linear form. The copoly-(succinimide-aspartic acid) formed by partial hydrolysis and especially polyaspartic acid (trade name Baypure® DS 100) produced by partial hydrolysis is suitable as a long-lasting inhibitor against limescale deposition in water treatment and applications in the oil and mining industries, and as a setting retarder for cement in the construction industry.[18] Patent literature[10] mentions polysuccinimide applications as chelating agents, inhibitors against scale formation, dispersant, humectants, and fertilizer additives.
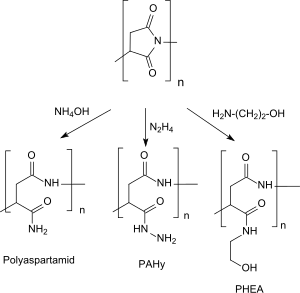
The opening of the pyrrolidinedione ring structures in polysuccinimide via aminolysis with ammonia water (containgin NH4OH) produces poly-(α, β)-DL-asparagine, with hydrazine poly-(α, β)-DL-aspartylhydrazide (PAHy) and with functional amines, e.g. ethanolamine poly-(α), β)-DL-2-hydroxyethylaspartate (PHEA).[8] PHEA can be used a plasma expander with good biocompatibility and biodegradability, high water solubility at low manufacturing costs and was investigated more intensive as a potential drug carrier) in medical applications.[19][20]
Cross-linked poly(α, β)-DL aspartic acid sodium salt, which is the commercially most interesting polysuccinimide derivative, has been extensively tested for its suitability as a biodegradable superabsorbent compared to the non-biodegradable standard cross-linked sodium polyacrylate.[21][22] The results obtained have not yet led to the use of crosslinked polyaspartic acid in large-volume applications for superabsorbents (e.g. baby diapers).
References
- E. Jalalvandi, A. Shavandi (2018), "Polysuccinimide and its derivatives: Degradable and water soluble polymers (review)", Eur. Polym. J., 109, pp. 43–54, doi:10.1016/j.eurpolymj.2018.08.056
- T. Klein, R.-J. Moritz, R. Graupner (2016), Ullmann‘s Polymers and Plastics, Products and Processes, Volume 1, Part 2: Organic Polymers, Polyaspartates and Polysuccinimide, Weinheim: Wiley-VCH, pp. 742–743, ISBN 978-3-527-33823-8CS1 maint: multiple names: authors list (link)
- M. Tomida, T. Nakato, M. Kuramochi, M. Shibata, S. Matsunami, T. Kakuchi (1996), "Novel method of synthesizig poly(succinimide) and its copolymeric derivatives by acid-catalysed polycondensation of L-aspartic acid", Polymer, 37 (16), pp. 4435–4437, doi:10.1016/0032-3861(96)00267-4CS1 maint: multiple names: authors list (link)
- Baypure® General Product Information (PDF) Lanxess AG
- Hugo Schiff (1897-09-01), "Ueber Polyaspartsäuren", Berichte der deutschen chemischen Gesellschaft (in German), 30 (3), pp. 2449–2459, doi:10.1002/cber.18970300316
- Kenneth Doll, Randal Shogren, Ronald Holser, J. Willett, Graham Swift (2005-12-01), "Polymerization of L-Aspartic Acid to Polysuccinimide and Copoly(Succinimide-Aspartate) in Supercritical Carbon Dioxide", Letters in Organic Chemistry, 2 (8), pp. 687–689, doi:10.2174/157017805774717553CS1 maint: multiple names: authors list (link)
- Thomas Klein, Ralf-Johann Moritz, René Graupner (2008), "Polyaspartates and Polysuccinimide", Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.l21_l01, ISBN 978-3-527-30673-2CS1 maint: multiple names: authors list (link)
- Paolo Neri, Guido Antoni, Franco Benvenuti, Francesco Cocola, Guido Gazzei (1973-08-01), "Synthesis of α, β-poly [(2-hydroxyethyl)-DL-aspartamide], a new plasma expander", Journal of Medicinal Chemistry, 16 (8), pp. 893–897, doi:10.1021/jm00266a006CS1 maint: multiple names: authors list (link)
- US 5142062, J. Knebel, K. Lehmann, "Method for increasing the molecular weight in the manufacture of polysuccinimide", issued 1992-08-25, assigned to Röhm GmbH
- EU 0791616, M. Uenaka et al., "Process for producing polysuccinimide and use of said compound", issued 1997-8-27, assigned to Mitsubishi Chemical Corp.
- US 5756595, G.Y. Mazo et al., "Catalytically polymerizing aspartic acid", issued 1998-05-26, assigned to Donlar Corp.
- US 7053170, C.S. Sikes, "Preparation of high molecular weight polysuccinimides", issued 2006-05-30, assigned to Aquero Co.
- EU 0612784, T. Groth et al., "Process for preparing polysuccinimide and polyaspartic acid", issued 1994-08-31, assigned to Bayer AG
- US 5296578, L.P. Koskan, A.R.Y. Meah, "Production of polysuccinimide and polyaspartic acid acid from maleic anhydride and ammonia", issued 1994-03-22, assigned to Donlar Corp.
- US 5393868, M. B. Freeman et al., "Production of polysuccinimide by thermal polymerization of maleamic acid", issued 1995-02-28, assigned to Rohm and Haas Co.
- K.C. Low et al.: 6. Commercial Poly(aspartic acid) and Its Uses. In: J.E. Glass: Hydrophilic Polymers, Advances in Chemistry. 248, 1996, ISBN 978-0-8412-3133-7, S. 99–111, doi:10.1021/ba-1996-0248.ch006.
- G. Swift: Degradable Polymers. 2nd ed. Springer Netherlands, 2002, S. 379–412, doi:10.1007/978-94-017-1217-0_11.
- T. Klein: Baypure®, An innovate product family for household and technical applications. 5th Green Chemistry Conference 2003, Barcelona.
- K. Seo, D. Kim: Design and synthesis of endosomolytic conjugated polyaspartamide for cytosolic drug delivery. In: E. Jabbari, A. Khademhosseini (Hrsg.): Biologically-responsive hybrid biomaterials: a reference for material scientists and bioengineers. World Scientific Publishing Co., Singapur 2010, ISBN 978-981-4295-67-3, S. 191–212, doi:10.1142/9789814295680_0009.
- Eberhard W. Neuse, Axel G. Perlwitz, Siegfried Schmitt (1991-11-01), "Water-soluble polyamides as potential drug carriers. III. Relative main-chain stabilities of side chain-functionalized aspartamide polymers on aqueous-phase dialysis", Die Angewandte Makromolekulare Chemie, 192 (1), pp. 35–50, doi:10.1002/apmc.1991.051920103CS1 maint: multiple names: authors list (link)
- US 5859179, Y. Chou, "Forming superabsorbent polymer", issued 1999-01-19, assigned to Solutia Inc.
- US 6072024, Y. Irizato et al., "Production process of cross-linked polyaspartic acid", issued 2000-06-06, assigned to Mitsui Chemicals