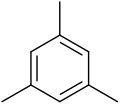
Mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colourless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.[4]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,5-Trimethylbenzene[1] | |||
| Other names
Mesitylene[1] sym-Trimethylbenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.278 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
| UN number | 2325 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H12 | |||
| Molar mass | 120.19 g/mol | ||
| Appearance | Clear, colorless liquid[2] | ||
| Odor | Distinctive, aromatic[2] | ||
| Density | 0.8637 g/cm3 at 20 °C | ||
| Melting point | −44.8 °C (−48.6 °F; 228.3 K) | ||
| Boiling point | 164.7 °C (328.5 °F; 437.8 K) | ||
| 0.002% (20°C)[2] | |||
| Vapor pressure | 2 mmHg (20°C)[2] | ||
| -92.32·10−6 cm3/mol | |||
| Structure | |||
| 0.047 D [3] | |||
| Hazards | |||
| Safety data sheet | Safety data from Sigma Aldrich | ||
| Flash point | 50 °C; 122 °F; 323 K[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[2] | ||
REL (Recommended) |
TWA 25 ppm (125 mg/m3)[2] | ||
IDLH (Immediate danger) |
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Mesitylene is prepared by equilibration of xylene (or simple methyl alkylation of it) over solid acid catalyst:[4]
- 2 C6H4(CH3)2 ⇌ C6H3(CH3)3 + C6H5CH3
- C6H4(CH3)2 + CH3OH → C6H3(CH3)3 + H2O
Trimerization of acetone via aldol condensation, which is catalyzed and dehydrated by sulfuric acid affords a mixture of 1,3,5- and 1,2,4-trimethylbenzenes.[5]
Reactions
Oxidation of mesitylene with nitric acid affords trimesic acid, C6H3(COOH)3. Using manganese dioxide, a milder oxidising agent, 3,5-dimethylbenzaldehyde is formed. Mesitylene is oxidised by trifluoroperacetic acid to produce mesitol (2,4,6-trimethylphenol).[6]
Applications
Mesitylene is mainly used as a precursor to 2,4,6-trimethylaniline, a precursor to colorants. This derivative is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups.[7]
Additive and component of some avgas (aviation gasoline) blends.
Niche uses
molybdenum_tricarbonyl.png.webp)
]
Mesitylene is used in the laboratory as a specialty solvent. It can also act as a ligand in organometallic chemistry, one example being the organomolybdenum complex [(η6-C6H3Me3)Mo(CO)3].[8] which can be prepared from molybdenum hexacarbonyl.
In the electronics industry, mesitylene has been used as a developer for photopatternable silicones due to its solvent properties.
The three aromatic hydrogen atoms of mesitylene are in identical chemical shift environments. Therefore, they only give a single peak near 6.8 ppm in the 1H NMR spectrum; the same is also true for the nine methyl protons, which give a singlet near 2.3 ppm. For this reason, mesitylene is sometimes used as an internal standard in NMR samples that contain aromatic protons.[9]
Uvitic acid is obtained by oxidizing mesitylene or by condensing pyruvic acid with baryta water.[10]
The Gattermann reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide (Zn(CN)2).[11] Although it is highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous hydrogen cyanide (HCN).[12] The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(CN)2 that serves as the Lewis-acid catalyst in-situ. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene.[13]
History
Mesitylene was first prepared in 1837 by Robert Kane, an Irish chemist, by heating acetone with concentrated sulfuric acid.[14] He named his new substance "mesitylene" because the German chemist Carl Reichenbach had named acetone "mesit" (from the Greek μεσίτης, the mediator),[15] and Kane believed that his reaction had dehydrated mesit, converting it to an alkene, "mesitylene".[16] However, Kane's determination of the chemical composition ("empirical formula") of mesitylene was incorrect. The correct empirical formula was provided by August W. von Hofmann in 1849.[17] In 1866 Adolf von Baeyer showed that mesitylene's structure was consistent with that of 1,3,5-trimethylbenzene;[18] however, conclusive proof that mesitylene was identical to 1,3,5-trimethylbenzene was provided by Albert Ladenburg in 1874.[19]
Mesityl group
The group (CH3)3C6H2- is called mesityl (organic group symbol: Mes). Mesityl derivatives, e.g. tetramesityldiiron, are typically prepared from the Grignard reagent (CH3)3C6H2MgBr. Due to its large steric demand, the mesityl group is used as a large blocking group in asymmetric catalysis (to enhance diastereo- or enantioselectivity) and organometallic chemistry (to stabilize low oxidation state or low coordination number metal centers). Larger analogues with even greater steric demand, for example 2,6-diisopropylphenyl (Dipp) and the analogously named Tripp ((iPr)3C6H2, Is) and supermesityl ((tBu)3C6H2, Mes*) groups, may be even more effective toward achieving these goals.
Safety and the environment
Mesitylene is also a major urban volatile organic compound (VOC) which results from combustion. It plays a significant role in aerosol and tropospheric ozone formation as well as other reactions in atmospheric chemistry.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 139. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0639". National Institute for Occupational Safety and Health (NIOSH).
- Zhao, Jun; Zhang, Renyi (2004). "Proton transfer reaction rate constants between hydronium ion (H3O+) and volatile organic compounds". Atmospheric Environment. 38 (14): 2177–2185. doi:10.1016/j.atmosenv.2004.01.019.
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke “Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227.
- Cumming, W. M. (1937). Systematic organic chemistry (3E). New York, USA: D. Van Nostrand Company. p. 57.
- Chambers, Richard D. (2004). "Functional Compounds Containing Oxygen, Sulphur or Nitrogen and their Derivatives". Fluorine in Organic Chemistry. CRC Press. pp. 242–243. ISBN 9780849317903.
- Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
- Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999. ISBN 0-93570248-2.
- http://chemicalland21.com/industrialchem/organic/MESITYLENE.htm
- "Definition of uvitic acid". merriam-webster.com. Retrieved 31 October 2016.
- Adams R.; Levine, I. (1923). "Simplification of the Gattermann Synthesis of Hydroxy Aldehydes". J. Am. Chem. Soc. 45 (10): 2373–77. doi:10.1021/ja01663a020.
- Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. pp. 38 & 53–54. doi:10.1002/0471264180.or009.02. ISBN 9780471007265.
- Fuson, R. C.; Horning, E. C.; Rowland, S. P.; Ward, M. L. (1955). "Mesitaldehyde". Organic Syntheses. doi:10.15227/orgsyn.023.0057.; Collective Volume, 3, p. 549
- Robert Kane (1839) "On a series of combinations derived from pyroacetic spirit [acetone]" Transactions of the Royal Irish Academy, vol. 18, pages 99–125.
- Reichenbach's research is excerpted in: C. Reichenbach (1834) "Ueber Mesit (Essiggeist) und Holzgeist" (On mesit (spirit of vinegar) and wood spirits), Annalen der Pharmacie, vol. 10, no. 3, pages 298–314.
- For an explanation of the original of the name "mesitylene", see also: Henry E. Roscoe, A Treatise on Chemistry (New York, New York: D. Appleton and Co., 1889), vol. III, page 102, footnote 2.
- A.W. Hofmann (1849) "On the composition of mesitilole [mesitylene], and some of its derivatives", The Quarterly Journal of the Chemical Society of London, vol. 2, pages 104–115. (Note: The empirical formula of mesitylene as stated in Hofmann's paper ( C18H12 ) is incorrect; however, this happened because Hofmann used 6 as the atomic weight of carbon, instead of the correct atomic weight of 12. Once the correct atomic weight is used in Hofmann's calculations, his results give the correct empirical formula of C9H12.)
- Adolf von Baeyer (1866) "Ueber die Condensationsproducte des Acetons" (On condensation products of acetone), Annalen der Chemie und Pharmacie, vol. 140, pages 297–306.
- Albert Ladenburg (1874) "Ueber das Mesitylen" (On mesitylene), Berichte der deutschen chemischen Gesellschaft, vol. 7, pages 1133–1137. doi: 10.1002/cber.18740070261