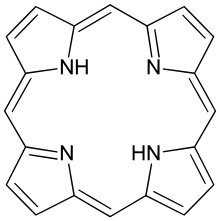
Porphine
Porphine or porphin is an organic chemical compound with formula C
20H
14N
4. The molecule consists of four pyrrole-like rings joined by four methine (=CH−) groups to form a larger macrocycle ring, which makes it the simplest of the tetrapyrroles. It is an aromatic and heterocyclic compound, solid at room temperature.[1]
 | |
 | |
| Names | |
|---|---|
| Other names
Porphin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.690 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H14N4 | |
| Molar mass | 310.35196 g/mol |
| Appearance | Dark red, shiny leaflets |
| Melting point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Porphine does not occur in nature, and is almost only of theoretical interest; however, substituted derivatives include many biochemically significant compounds called porphyrins, with the dominant example being protoporphyrin IX.[2] Many synthetic analogues are also known, including octaethylporphyrin[3] and tetraphenylporphyrin.[4]
Structural characteristics of porphine and its derivatives
- Common porphyrins
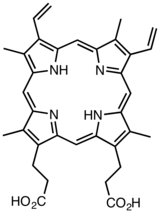 Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.
Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.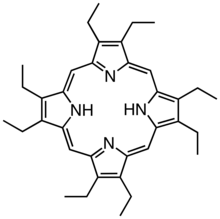 Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.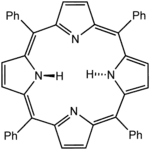 Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methine centers are occupied by phenyl groups.
Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methine centers are occupied by phenyl groups.
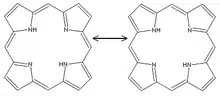
Porphine is planar. The two NH centers are trans.
Complex with metal in the center
Concomitant with the displacement of two N-H protons, porphine binds metal ions at its center. The insertion of the metal center is slow in the absence of catalysts. In nature, these catalysts (enzymes) are called chelatases. When there is no metal ion (or atom) bound to the nitrogens in the center, then the compounds are called free porphine or free porphyrin. If they are bonded to a metal in the center, then they are bound. A porphyrin with an iron atom of the type found in myoglobin, hemoglobin, or certain cytochromes is called heme. See the Porphyrin article for further details.
Compounds similar to porphine are the parent compounds for similar ring systems with other central metal atoms in biochemistry. These include chlorin, which has a magnesium ion in several types of chlorophyll; bacteriochlorin, found in bacteriochlorophyll; and corrin, which has a cobalt center in cobalamin (vitamin B12).
See also
- Tetrahydroporphine
Further reading
- Budavari, Susan (1989). "7574. Porphine". The Merck Index (11th ed.). Merck & Co., Inc. p. 1210. ISBN 0-911910-28-X. LCCN 89-60001.
References
- "Porphyrin". Encyclopedia of Inorganic and Bioinorganic Chemistry. Wiley-VCH. 2011. doi:10.1002/9781119951438.eibd0638. ISBN 9781119951438.
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- Jonathan L. Sessler; Azadeh Mozaffari; Martin R. Johnson (1992). "3,4-Diethylpyrrole and 2,3,7,8,12,13,17,18-Octaethylporphyrin". Org. Synth. 70: 68. doi:10.15227/orgsyn.070.0068.
- Lindsey, Jonathan S. (2000). "Synthesis of meso-substituted porphyrins". In Kadish, Karl M.; Smith, Kevin M.; Guilard, Roger (eds.). Porphyrin Handbook. 1. pp. 45–118. ISBN 0-12-393200-9.