Pyruvate decarboxylation
Pyruvate decarboxylation or pyruvate oxidation, also known as the link reaction, is the conversion of pyruvate into acetyl-CoA by the enzyme complex pyruvate dehydrogenase complex.[1][2]
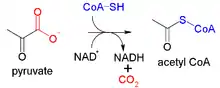
The reaction may be simplified as:
1 Pyruvate + 1 NAD+ + CoA → 1 Acetyl-CoA + NADH + CO2 + H+
Pyruvate oxidation is the step that connects glycolysis and the Krebs cycle.[3] In glycolysis, a single glucose molecule (6 carbons) is split into 2 pyruvates (3 carbons each). Because of this, the link reaction occurs twice for each glucose molecule to produce a total of 2 acetyl-CoA molecules, which can then enter the Krebs cycle.
Energy-generating ions and molecules, such as amino acids and carbohydrates, enter the Krebs cycle as acetyl coenzyme A and oxidize in the cycle.[4] The pyruvate dehydrogenase complex (PDC) catalyzes the decarboxylation of pyruvate, resulting in the synthesis of acetyl-CoA, CO2, and NADH. In eukaryotes, this enzyme complex regulates pyruvate metabolism, and ensures homeostasis of glucose during absorptive and post-absorptive state metabolism.[5] As the Krebs cycle occurs in the mitochondrial matrix, the pyruvate generated during glycolysis in the cytosol is transported across the inner mitochondrial membrane by a pyruvate carrier under aerobic conditions.
References
- "Pyruvate oxidation". Khanacademy.org. Retrieved 25 January 2018.
- "Pyruvate Oxidation". Oregonstate.edu. Retrieved 25 January 2018.
- Trifiletti, R. R. (2014-01-01), Aminoff, Michael J.; Daroff, Robert B. (eds.), "Thiamine (Vitamin B1) and Beri-Beri", Encyclopedia of the Neurological Sciences (Second Edition), Oxford: Academic Press, pp. 445–447, doi:10.1016/b978-0-12-385157-4.00116-0, ISBN 978-0-12-385158-1, retrieved 2020-11-16
- Stryer, Lubert; Tymoczko, John L.; Berg, Jeremy M. (2002). "The Citric Acid Cycle". Biochemistry. 5th edition.
- Jordan, Frank; Furey, William; Nemeria, Natalia S.; Patel, Mulchand S. (2014-06-13). "The Pyruvate Dehydrogenase Complexes: Structure-based Function and Regulation". Journal of Biological Chemistry. 289 (24): 16615–16623. doi:10.1074/jbc.R114.563148. ISSN 1083-351X. PMC 4059105. PMID 24798336.