Quality of life (healthcare)
In general, quality of life (QoL or QOL) is the perceived quality of an individual's daily life, that is, an assessment of their well-being or lack thereof. This includes all emotional, social and physical aspects of the individual's life. In health care, health-related quality of life (HRQoL) is an assessment of how the individual's well-being may be affected over time by a disease, disability or disorder.[1][2]
Measurement
Early versions of healthcare-related quality of life measures referred to simple assessments of physical abilities by an external rater (for example, the patient is able to get up, eat and drink, and take care of personal hygiene without any help from others) or even to a single measurement (for example, the angle to which a limb could be flexed).
The current concept of health-related quality of life acknowledges that subjects put their actual situation in relation to their personal expectation.[3] The latter can vary over time, and react to external influences such as length and severity of illness, family support, etc. As with any situation involving multiple perspectives, patients' and physicians' rating of the same objective situation have been found to differ significantly. Consequently, health-related quality of life is now usually assessed using patient questionnaires. These are often multidimensional and cover physical, social, emotional, cognitive, work- or role-related, and possibly spiritual aspects as well as a wide variety of disease related symptoms, therapy induced side effects, and even the financial impact of medical conditions.[4] Although often used interchangeably with the measurement of health status, both health-related quality of life and health status measure different concepts.
Activities of daily living
Because health problems can interfere with even the most basic aspects of daily living (for example, breathing comfortably, quality of sleep, eliminating wastes, feeding oneself, dressing, and others), the health care professions have codified the concepts of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). Such analysis and classification helps to at least partially objectify quality of life. It cannot eliminate all subjectivity, but it can help improve measurement and communication by quantifying and by reducing ineffability.
Examples
Similar to other psychometric assessment tools, health-related quality of life questionnaires should meet certain quality criteria, most importantly with regard to their reliability and validity. Hundreds of validated health-related quality of life questionnaires have been developed, some of which are specific to various illnesses. The questionnaires can be generalized into two categories:
Generic instruments
- CDC HRQOL–14 "Healthy Days Measure": A questionnaire with four base questions and ten optional questions used by the Center for Disease Control and Prevention (CDC) (https://www.cdc.gov/hrqol/hrqol14_measure.htm).
- Short-Form Health Survey (SF-36, SF-12, SF-8): One example of a widely used questionnaire assessing physical and mental health-related quality of life. Used in clinical trials and population health assessments. Suitable for pharmacoeconomic analysis, benefiting healthcare rationing.
- EQ-5D a simple quality of life questionnaire (https://euroqol.org).
- AQoL-8D a comprehensive questionnaire [5][6] that assesses HR-QoL over 8 domains - independent living, happiness, mental health, coping, relationships, self-worth, pain, senses (https://www.aqol.com.au).
Disease, disorder or condition specific instruments
- King's Health Questionnaire (KHQ)
- International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) in urinary incontinence,[7] the LC -13 Lung Cancer module from the EORTC Quality of Life questionnaire library, or the Hospital Anxiety and Depression Scale (HADS) ).
- Manchester Short Assessment of Quality of Life: 16-item questionnaire for use in psychiatric populations.
- ECOG, most commonly used to evaluate the impact of cancer on sufferers.
- NYHA scale, most commonly used to evaluate the impact of heart disease on individuals.
- EORTC measurement system for use in clinical trials in oncology.[8] These tools are robustly tested and validated[9] and translated.[10] A large amount of reference data is now available.[11] The field of HRQOL has grown significantly in the last decade, with hundreds of new studies and better reporting of clinical trials.[12] HRQOL appears to be prognostic for survival in some diseases and patients.[13][14]
- WHO-Quality of life-BREF (WHOQOL-BREF): A general Quality of life survey validated for several countries.[15]
- The Stroke Specific Quality Of Life scale SS-QOL: It is a patient-centered outcome measure intended to provide an assessment of health-related quality of life (HRQOL) specific to patients with stroke only. It measures energy, family roles, language, mobility, mood, personality, self care, social roles, thinking, upper extremity function, vision and work productivity.[16]
- In rheumatology, condition specific instruments have been developed such as RAQoL[17] for rheumatoid arthritis, OAQoL[18] for osteoarthritis, ASQoL[19] for ankylosing spondylitis, SScQoL[20] for systemic sclerosis and PsAQoL[21] for people with psoriatic arthritis.
Utility
A variety of validated surveys exist for healthcare providers to use for measuring a patient’s health-related quality of life. The results are then used to help determine treatment options for the patient based on past results from other patients,[22] and to measure intra-individual improvements in QoL pre- and post-treatment.
When it is used as a longitudinal study device that surveys patients before, during, and after treatment, it can help health care providers determine which treatment plan is the best option, thereby improving healthcare through an evolutionary process.
Importance
There is a growing field of research concerned with developing, evaluating, and applying quality of life measures within health related research (e.g. within randomized controlled studies), especially in relation to Health Services Research. Well-executed health-related quality of life research informs those tasked with health rationing or anyone involved in the decision-making process of agencies such as the Food and Drug Administration, European Medicines Agency[23] or National Institute for Clinical Excellence.[24] Additionally, health-related quality of life research may be used as the final step in clinical trials of experimental therapies.
The understanding of Quality of Life is recognized as an increasingly important healthcare topic because the relationship between cost and value raises complex problems, often with high emotional attachment because of the potential impact on human life. For instance, healthcare providers must refer to cost-benefit analysis to make economic decisions about access to expensive drugs that may prolong life by a short amount of time and/or provide a minimal increase to quality of life. Additionally, these treatment drugs must be weighed against the cost of alternative treatments or preventative medicine. In the case of chronic and/or terminal illness where no effective cure is available, an emphasis is placed on improving health-related quality of life through interventions such as symptom management,[25] adaptive technology, and palliative care.
In the realm of elder care, research indicates that improvements in quality of life ratings may also improve resident outcomes, which can lead to substantial cost savings over time. Research has also shown that quality of life ratings can be successfully used as a key-performance metric when designing and implementing organizational change initiatives in nursing homes.[26]
Research
Research revolving around Health Related Quality of Life is extremely important because of the implications that it can have on current and future treatments and health protocols. Thereby, validated health-related quality of life questionnaires can become an integral part of clinical trials in determining the trial drugs' value in a cost-benefit analysis. For example, the Center for Disease Control and Prevention (CDC) is using their health-related quality of life survey, Healthy Day Measure, as part of research to identify health disparities, track population trends, and build broad coalitions around a measure of population health. This information can then be used by multiple levels of government or other officials to "increase quality and years of life" and to "eliminate health disparaties" for equal opportunity. [27]
Ethics
The quality of life ethic refers to an ethical principle that uses assessments of the quality of life that a person could potentially experience as a foundation for making decisions about the continuation or termination of life. It is often used in contrast to or in opposition to the sanctity of life ethic.
Analysis
Statistical biases
It is not considered uncommon for there to be some statistical anomalies during data analysis. Some of the more frequently seen in health-related quality of life analysis are the ceiling effect, the floor effect, and response shift bias.
The ceiling effect refers to how patients who start with a higher quality of life than the average patient do not have much room for improvement when treated. The opposite of this is the floor effect, where patients with a lower quality of life average have much more room for improvement.[3] Consequentially, if the spectrum of quality of life before treatment is too unbalanced, there is a greater potential for skewing the end results, creating the possibility for incorrectly portraying a treatment's effectiveness or lack thereof.
Response shift bias
Response shift bias is an increasing problem within longitudinal studies that rely on patient reported outcomes.[28] It refers to the potential of a subject’s views, values, or expectations changing over the course of a study, thereby adding another factor of change on the end results. Clinicians and healthcare providers must recalibrate surveys over the course of a study to account for Response Shift Bias.[29] The degree of recalibration varies due to factors based on the individual area of investigation and length of study.
Statistical variation
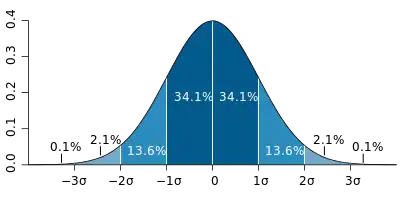
In a study by Norman et al. about health-related quality of life surveys, it was found that most survey results were within a half standard deviation. Norman et al. theorized that this is due to the limited human discrimination ability as identified by George A. Miller in 1956. Utilizing the Magic Number of 7 ± 2, Miller theorized that when the scale on a survey extends beyond 7 ± 2, humans fail to be consistent and lose ability to differentiate individual steps on the scale because of channel capacity.
Norman et al. proposed health-related quality of life surveys use a half standard deviation as the statistically significant benefit of a treatment instead of calculating survey-specific “minimally important differences", which are the supposed real-life improvements reported by the subjects.[30] In other words, Norman et al. proposed all health-related quality of life survey scales be set to a half standard deviation instead of calculating a scale for each survey validation study where the steps are referred to as "minimally important differences".
References
- CDC - Concept - Health-Related Quality of Life
- Bottomley A (April 2002). "The cancer patient and quality of life". The Oncologist. 7 (2): 120–5. doi:10.1634/theoncologist.7-2-120. PMID 11961195.
- Jongen PJ, Lehnick D, Sanders E, Seeldrayers P, Fredrikson S, Andersson M, Speck J (November 2010). "Health-related quality of life in relapsing remitting multiple sclerosis patients during treatment with glatiramer acetate: a prospective, observational, international, multi-centre study". Health and Quality of Life Outcomes. 8: 133. doi:10.1186/1477-7525-8-133. PMC 2999586. PMID 21078142.
- Burckhardt CS, Anderson KL (October 2003). "The Quality of Life Scale (QOLS): reliability, validity, and utilization". Health and Quality of Life Outcomes. 1: 60. doi:10.1186/1477-7525-1-60. PMC 269997. PMID 14613562.
- Richardson J, Iezzi A, Khan MA, Maxwell A (2014). "Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument". The Patient. 7 (1): 85–96. doi:10.1007/s40271-013-0036-x. PMC 3929769. PMID 24271592.
- Maxwell A, Özmen M, Iezzi A, Richardson J (December 2016). "Deriving population norms for the AQoL-6D and AQoL-8D multi-attribute utility instruments from web-based data". Quality of Life Research. 25 (12): 3209–3219. doi:10.1007/s11136-016-1337-z. PMID 27344318. S2CID 2153470.
- Hirakawa T, Suzuki S, Kato K, Gotoh M, Yoshikawa Y (August 2013). "Randomized controlled trial of pelvic floor muscle training with or without biofeedback for urinary incontinence". International Urogynecology Journal. 24 (8): 1347–54. doi:10.1007/s00192-012-2012-8. PMID 23306768. S2CID 19485395.
- Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, Velikova G, Blazeby J, Greimel E (August 2005). "Health related quality of life outcomes in cancer clinical trials". European Journal of Cancer. 41 (12): 1697–709. doi:10.1016/j.ejca.2005.05.007. PMID 16043345.
- Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, Velikova G, Blazeby J, Greimel E (August 2005). "Health related quality of life outcomes in cancer clinical trials". European Journal of Cancer. 41 (12): 1697–709. doi:10.1016/j.ejca.2005.05.007. PMID 16043345.
- Koller M, Kantzer V, Mear I, Zarzar K, Martin M, Greimel E, Bottomley A, Arnott M, Kuliś D (April 2012). "The process of reconciliation: evaluation of guidelines for translating quality-of-life questionnaires". Expert Review of Pharmacoeconomics & Outcomes Research. 12 (2): 189–97. doi:10.1586/erp.11.102. PMID 22458620. S2CID 207222200.
- EORTC Quality of Life Group = (July 2008). EORTC QLQ-C30 Reference Values (PDF). Retrieved 4 May 2015.
- Velikova, G.; Coens, C.; Efficace, F.; Greimel, E.; Groenvold, M.; Johnson, C.; Singer, S.; Van De Poll-Franse, L.; Young, T.; Bottomley, A. (2012). "Health-Related Quality of Life in EORTC clinical trials — 30 years of progress from methodological developments to making a real impact on oncology practice". European Journal of Cancer Supplements. 10: 141–149. doi:10.1016/S1359-6349(12)70023-X.
- Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, Bottomley A (September 2009). "Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials". The Lancet. Oncology. 10 (9): 865–71. doi:10.1016/S1470-2045(09)70200-1. PMID 19695956.
- Weis J, Arraras JI, Conroy T, Efficace F, Fleissner C, Görög A, Hammerlid E, Holzner B, Jones L, Lanceley A, Singer S, Wirtz M, Flechtner H, Bottomley A (May 2013). "Development of an EORTC quality of life phase III module measuring cancer-related fatigue (EORTC QLQ-FA13)". Psycho-Oncology. 22 (5): 1002–7. doi:10.1002/pon.3092. PMID 22565359.
- "WHO Quality of Life-BREF (WHOQOL-BREF)". WHO. Retrieved 4 May 2015..
- Silva SM, Corrêa FI, Faria CD, Corrêa JC (February 2015). "Psychometric properties of the stroke specific quality of life scale for the assessment of participation in stroke survivors using the rasch model: a preliminary study". Journal of Physical Therapy Science. 27 (2): 389–92. doi:10.1589/jpts.27.389. PMC 4339145. PMID 25729175.
- Whalley D, McKenna SP, de Jong Z, van der Heijde D (August 1997). "Quality of life in rheumatoid arthritis". British Journal of Rheumatology. 36 (8): 884–8. doi:10.1093/rheumatology/36.8.884. PMID 9291858.
- Keenan AM, McKenna SP, Doward LC, Conaghan PG, Emery P, Tennant A (June 2008). "Development and validation of a needs-based quality of life instrument for osteoarthritis". Arthritis and Rheumatism. 59 (6): 841–8. doi:10.1002/art.23714. PMID 18512719.
- Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, McKenna SP, Tennant A, van der Heijde D, Chamberlain MA (January 2003). "Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis". Annals of the Rheumatic Diseases. 62 (1): 20–6. doi:10.1136/ard.62.1.20. PMC 1754293. PMID 12480664.
- Ndosi M, Alcacer-Pitarch B, Allanore Y, Del Galdo F, Frerix M, García-Díaz S, Hesselstrand R, Kendall C, Matucci-Cerinic M, Mueller-Ladner U, Sandqvist G, Torrente-Segarra V, Schmeiser T, Sierakowska M, Sierakowska J, Sierakowski S, Redmond A (February 2018). "Common measure of quality of life for people with systemic sclerosis across seven European countries: a cross-sectional study". Annals of the Rheumatic Diseases. 77 (7): annrheumdis–2017–212412. doi:10.1136/annrheumdis-2017-212412. PMC 6029637. PMID 29463517.
- McKenna SP, Doward LC, Whalley D, Tennant A, Emery P, Veale DJ (February 2004). "Development of the PsAQoL: a quality of life instrument specific to psoriatic arthritis". Annals of the Rheumatic Diseases. 63 (2): 162–9. doi:10.1136/ard.2003.006296. PMC 1754880. PMID 14722205.
- "Health Measures Quality of Life". www.healthypeople.gov. Retrieved 30 September 2017.
- Bottomley A, Jones D, Claassens L (February 2009). "Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency". European Journal of Cancer. 45 (3): 347–53. doi:10.1016/j.ejca.2008.09.032. PMID 19013787.
- Marquis P, Caron M, Emery MP, et al. (2011). "The Role of Health-Related Quality of Life Data in the Drug Approval Processes in the US and Europe: A Review of Guidance Documents and Authorizations of Medicinal Products from 2006 to 2010". Pharm Med. 25 (3): 147–60. doi:10.1007/bf03256856. S2CID 31174846.
- "Symptom management". NCI Dictionary of Cancer Terms. United States National Cancer Institute. 2011-02-02.
- Mitchell JM, Kemp BJ (March 2000). "Quality of life in assisted living homes: a multidimensional analysis". The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 55 (2): P117–27. doi:10.1093/geronb/55.2.P117. PMID 10794190.
- Testa MA, Nackley JF (1994). "Methods for quality-of-life studies". Annual Review of Public Health. 15: 535–59. doi:10.1146/annurev.pu.15.050194.002535. PMID 8054098.
- Ring L, Höfer S, Heuston F, Harris D, O'Boyle CA (September 2005). "Response shift masks the treatment impact on patient reported outcomes (PROs): the example of individual quality of life in edentulous patients". Health and Quality of Life Outcomes. 3: 55. doi:10.1186/1477-7525-3-55. PMC 1236951. PMID 16146573.
- Wagner JA (June 2005). "Response shift and glycemic control in children with diabetes". Health and Quality of Life Outcomes. 3: 38. doi:10.1186/1477-7525-3-38. PMC 1180844. PMID 15955236.
- Norman GR, Sloan JA, Wyrwich KW (May 2003). "Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation". Medical Care. 41 (5): 582–92. doi:10.1097/01.MLR.0000062554.74615.4C. PMID 12719681. S2CID 9198927.
External links
- ProQolid (Patient-Reported Outcome & Quality of Life Instruments Database)
- Mapi Research Trust ("Non-profit organization involved in Patient-Centered Outcomes")
- PROLabels(Database on Patient-Reported Outcome claims in marketing authorizations)
- Quality-of-Life-Recorder (Project to bring QoL measurement to routine practice. Platform & library of electronic questionnaires, Shareware/Freeware)
- The International Society for Quality of Life
- Health and Quality of Life Outcomes
- The Healthcare Center. Better Health for Everyone