Randomized controlled trial
A randomized controlled trial (or randomized control trial;[2] RCT) is a type of scientific (often medical) experiment that aims to reduce certain sources of bias when testing the effectiveness of new treatments; this is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response. One group—the experimental group—receives the intervention being assessed, while the other—usually called the control group—receives an alternative treatment, such as a placebo or no intervention. The groups are monitored under conditions of the trial design to determine the effectiveness of the experimental intervention, and efficacy is assessed in comparison to the control. [3] There may be more than one treatment group or more than one control group.
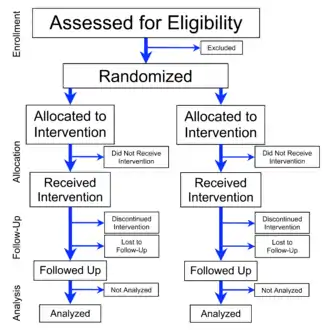
The trial may be blinded, meaning that information which may influence the participants is withheld until after the experiment is complete. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. Effective blinding may reduce or eliminate some sources of experimental bias.
The randomness in the assignment of subjects to groups reduces selection bias and allocation bias, balancing both known and unknown prognostic factors, in the assignment of treatments.[4] Blinding reduces other forms of experimenter and subject biases.
A well-blinded RCT is often considered the gold standard for clinical trials. Blinded RCTs are commonly used to test the efficacy of medical interventions and may additionally provide information about adverse effects, such as drug reactions. A randomized controlled trial can provide compelling evidence that the study treatment causes an effect on human health.[5]
The terms "RCT" and "randomized trial" are sometimes used synonymously, but the latter term omits mention of controls and can therefore describe studies that compare multiple treatment groups with each other in the absence of a control group.[6] Similarly, the initialism is sometimes expanded as "randomized clinical trial" or "randomized comparative trial", leading to ambiguity in the scientific literature.[7][8] Not all randomized clinical trials are randomized controlled trials (and some of them could never be, as in cases where controls would be impractical or unethical to institute). The term randomized controlled clinical trial is an alternative term used in clinical research;[9] however, RCTs are also employed in other research areas, including many of the social sciences.
History
The first reported clinical trial was conducted by James Lind in 1747 to identify treatment for scurvy.[10] Randomized experiments appeared in psychology, where they were introduced by Charles Sanders Peirce and Joseph Jastrow in the 1880s,[11] and in education.[12][13][14] Later, in the early 20th century, randomized experiments appeared in agriculture, due to Jerzy Neyman[15] and Ronald A. Fisher. Fisher's experimental research and his writings popularized randomized experiments.[16]
The first published RCT in medicine appeared in the 1948 paper entitled "Streptomycin treatment of pulmonary tuberculosis", which described a Medical Research Council investigation.[17][18][19] One of the authors of that paper was Austin Bradford Hill, who is credited as having conceived the modern RCT.[20]
Trial design was further influenced by the large-scale ISIS trials on heart attack treatments that were conducted in the 1980s.[21]
By the late 20th century, RCTs were recognized as the standard method for "rational therapeutics" in medicine.[22] As of 2004, more than 150,000 RCTs were in the Cochrane Library.[20] To improve the reporting of RCTs in the medical literature, an international group of scientists and editors published Consolidated Standards of Reporting Trials (CONSORT) Statements in 1996, 2001 and 2010, and these have become widely accepted.[1][4] Randomization is the process of assigning trial subjects to treatment or control groups using an element of chance to determine the assignments in order to reduce the bias.
Ethics
Although the principle of clinical equipoise ("genuine uncertainty within the expert medical community... about the preferred treatment") common to clinical trials[23] has been applied to RCTs, the ethics of RCTs have special considerations. For one, it has been argued that equipoise itself is insufficient to justify RCTs.[24] For another, "collective equipoise" can conflict with a lack of personal equipoise (e.g., a personal belief that an intervention is effective).[25] Finally, Zelen's design, which has been used for some RCTs, randomizes subjects before they provide informed consent, which may be ethical for RCTs of screening and selected therapies, but is likely unethical "for most therapeutic trials."[26][27]
Although subjects almost always provide informed consent for their participation in an RCT, studies since 1982 have documented that RCT subjects may believe that they are certain to receive treatment that is best for them personally; that is, they do not understand the difference between research and treatment.[28][29] Further research is necessary to determine the prevalence of and ways to address this "therapeutic misconception".[29]
The RCT method variations may also create cultural effects that have not been well understood.[30] For example, patients with terminal illness may join trials in the hope of being cured, even when treatments are unlikely to be successful.
Trial registration
In 2004, the International Committee of Medical Journal Editors (ICMJE) announced that all trials starting enrolment after July 1, 2005 must be registered prior to consideration for publication in one of the 12 member journals of the committee.[31] However, trial registration may still occur late or not at all.[32][33] Medical journals have been slow in adapting policies requiring mandatory clinical trial registration as a prerequisite for publication.[34]
Classifications
By study design
One way to classify RCTs is by study design. From most to least common in the healthcare literature, the major categories of RCT study designs are:[35]
- Parallel-group – each participant is randomly assigned to a group, and all the participants in the group receive (or do not receive) an intervention.[36][37]
- Crossover – over time, each participant receives (or does not receive) an intervention in a random sequence.[38][39]
- Cluster – pre-existing groups of participants (e.g., villages, schools) are randomly selected to receive (or not receive) an intervention.[40][41]
- Factorial – each participant is randomly assigned to a group that receives a particular combination of interventions or non-interventions (e.g., group 1 receives vitamin X and vitamin Y, group 2 receives vitamin X and placebo Y, group 3 receives placebo X and vitamin Y, and group 4 receives placebo X and placebo Y).
An analysis of the 616 RCTs indexed in PubMed during December 2006 found that 78% were parallel-group trials, 16% were crossover, 2% were split-body, 2% were cluster, and 2% were factorial.[35]
By outcome of interest (efficacy vs. effectiveness)
RCTs can be classified as "explanatory" or "pragmatic."[42] Explanatory RCTs test efficacy in a research setting with highly selected participants and under highly controlled conditions.[42] In contrast, pragmatic RCTs (pRCTs) test effectiveness in everyday practice with relatively unselected participants and under flexible conditions; in this way, pragmatic RCTs can "inform decisions about practice."[42]
By hypothesis (superiority vs. noninferiority vs. equivalence)
Another classification of RCTs categorizes them as "superiority trials", "noninferiority trials", and "equivalence trials", which differ in methodology and reporting.[43] Most RCTs are superiority trials, in which one intervention is hypothesized to be superior to another in a statistically significant way.[43] Some RCTs are noninferiority trials "to determine whether a new treatment is no worse than a reference treatment."[43] Other RCTs are equivalence trials in which the hypothesis is that two interventions are indistinguishable from each other.[43]
Randomization
The advantages of proper randomization in RCTs include:[44]
- "It eliminates bias in treatment assignment," specifically selection bias and confounding.
- "It facilitates blinding (masking) of the identity of treatments from investigators, participants, and assessors."
- "It permits the use of probability theory to express the likelihood that any difference in outcome between treatment groups merely indicates chance."
There are two processes involved in randomizing patients to different interventions. First is choosing a randomization procedure to generate an unpredictable sequence of allocations; this may be a simple random assignment of patients to any of the groups at equal probabilities, may be "restricted", or may be "adaptive." A second and more practical issue is allocation concealment, which refers to the stringent precautions taken to ensure that the group assignment of patients are not revealed prior to definitively allocating them to their respective groups. Non-random "systematic" methods of group assignment, such as alternating subjects between one group and the other, can cause "limitless contamination possibilities" and can cause a breach of allocation concealment.[45]
However empirical evidence that adequate randomization changes outcomes relative to inadequate randomization has been difficult to detect.[46]
Procedures
The treatment allocation is the desired proportion of patients in each treatment arm.
An ideal randomization procedure would achieve the following goals:[47]
- Maximize statistical power, especially in subgroup analyses. Generally, equal group sizes maximize statistical power, however, unequal groups sizes may be more powerful for some analyses (e.g., multiple comparisons of placebo versus several doses using Dunnett's procedure[48] ), and are sometimes desired for non-analytic reasons (e.g., patients may be more motivated to enroll if there is a higher chance of getting the test treatment, or regulatory agencies may require a minimum number of patients exposed to treatment).[49]
- Minimize selection bias. This may occur if investigators can consciously or unconsciously preferentially enroll patients between treatment arms. A good randomization procedure will be unpredictable so that investigators cannot guess the next subject's group assignment based on prior treatment assignments. The risk of selection bias is highest when previous treatment assignments are known (as in unblinded studies) or can be guessed (perhaps if a drug has distinctive side effects).
- Minimize allocation bias (or confounding). This may occur when covariates that affect the outcome are not equally distributed between treatment groups, and the treatment effect is confounded with the effect of the covariates (i.e., an "accidental bias"[44][50]). If the randomization procedure causes an imbalance in covariates related to the outcome across groups, estimates of effect may be biased if not adjusted for the covariates (which may be unmeasured and therefore impossible to adjust for).
However, no single randomization procedure meets those goals in every circumstance, so researchers must select a procedure for a given study based on its advantages and disadvantages.
Simple
This is a commonly used and intuitive procedure, similar to "repeated fair coin-tossing."[44] Also known as "complete" or "unrestricted" randomization, it is robust against both selection and accidental biases. However, its main drawback is the possibility of imbalanced group sizes in small RCTs. It is therefore recommended only for RCTs with over 200 subjects.[51]
Restricted
To balance group sizes in smaller RCTs, some form of "restricted" randomization is recommended.[51] The major types of restricted randomization used in RCTs are:
- Permuted-block randomization or blocked randomization: a "block size" and "allocation ratio" (number of subjects in one group versus the other group) are specified, and subjects are allocated randomly within each block.[45] For example, a block size of 6 and an allocation ratio of 2:1 would lead to random assignment of 4 subjects to one group and 2 to the other. This type of randomization can be combined with "stratified randomization", for example by center in a multicenter trial, to "ensure good balance of participant characteristics in each group."[4] A special case of permuted-block randomization is random allocation, in which the entire sample is treated as one block.[45] The major disadvantage of permuted-block randomization is that even if the block sizes are large and randomly varied, the procedure can lead to selection bias.[47] Another disadvantage is that "proper" analysis of data from permuted-block-randomized RCTs requires stratification by blocks.[51]
- Adaptive biased-coin randomization methods (of which urn randomization is the most widely known type): In these relatively uncommon methods, the probability of being assigned to a group decreases if the group is overrepresented and increases if the group is underrepresented.[45] The methods are thought to be less affected by selection bias than permuted-block randomization.[51]
Adaptive
At least two types of "adaptive" randomization procedures have been used in RCTs, but much less frequently than simple or restricted randomization:
- Covariate-adaptive randomization, of which one type is minimization: The probability of being assigned to a group varies in order to minimize "covariate imbalance."[51] Minimization is reported to have "supporters and detractors"[45] because only the first subject's group assignment is truly chosen at random, the method does not necessarily eliminate bias on unknown factors.[4]
- Response-adaptive randomization, also known as outcome-adaptive randomization: The probability of being assigned to a group increases if the responses of the prior patients in the group were favorable.[51] Although arguments have been made that this approach is more ethical than other types of randomization when the probability that a treatment is effective or ineffective increases during the course of an RCT, ethicists have not yet studied the approach in detail.[52]
Allocation concealment
"Allocation concealment" (defined as "the procedure for protecting the randomization process so that the treatment to be allocated is not known before the patient is entered into the study") is important in RCTs.[53] In practice, clinical investigators in RCTs often find it difficult to maintain impartiality. Stories abound of investigators holding up sealed envelopes to lights or ransacking offices to determine group assignments in order to dictate the assignment of their next patient.[45] Such practices introduce selection bias and confounders (both of which should be minimized by randomization), possibly distorting the results of the study.[45] Adequate allocation concealment should defeat patients and investigators from discovering treatment allocation once a study is underway and after the study has concluded. Treatment related side-effects or adverse events may be specific enough to reveal allocation to investigators or patients thereby introducing bias or influencing any subjective parameters collected by investigators or requested from subjects.
Some standard methods of ensuring allocation concealment include sequentially numbered, opaque, sealed envelopes (SNOSE); sequentially numbered containers; pharmacy controlled randomization; and central randomization.[45] It is recommended that allocation concealment methods be included in an RCT's protocol, and that the allocation concealment methods should be reported in detail in a publication of an RCT's results; however, a 2005 study determined that most RCTs have unclear allocation concealment in their protocols, in their publications, or both.[54] On the other hand, a 2008 study of 146 meta-analyses concluded that the results of RCTs with inadequate or unclear allocation concealment tended to be biased toward beneficial effects only if the RCTs' outcomes were subjective as opposed to objective.[55]
Sample size
The number of treatment units (subjects or groups of subjects) assigned to control and treatment groups, affects an RCT's reliability. If the effect of the treatment is small, the number of treatment units in either group may be insufficient for rejecting the null hypothesis in the respective statistical test. The failure to reject the null hypothesis would imply that the treatment shows no statistically significant effect on the treated in a given test. But as the sample size increases, the same RCT may be able to demonstrate a significant effect of the treatment, even if this effect is small.[56]
Blinding
An RCT may be blinded, (also called "masked") by "procedures that prevent study participants, caregivers, or outcome assessors from knowing which intervention was received."[55] Unlike allocation concealment, blinding is sometimes inappropriate or impossible to perform in an RCT; for example, if an RCT involves a treatment in which active participation of the patient is necessary (e.g., physical therapy), participants cannot be blinded to the intervention.
Traditionally, blinded RCTs have been classified as "single-blind", "double-blind", or "triple-blind"; however, in 2001 and 2006 two studies showed that these terms have different meanings for different people.[57][58] The 2010 CONSORT Statement specifies that authors and editors should not use the terms "single-blind", "double-blind", and "triple-blind"; instead, reports of blinded RCT should discuss "If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how."[4]
RCTs without blinding are referred to as "unblinded",[59] "open",[60] or (if the intervention is a medication) "open-label".[61] In 2008 a study concluded that the results of unblinded RCTs tended to be biased toward beneficial effects only if the RCTs' outcomes were subjective as opposed to objective;[55] for example, in an RCT of treatments for multiple sclerosis, unblinded neurologists (but not the blinded neurologists) felt that the treatments were beneficial.[62] In pragmatic RCTs, although the participants and providers are often unblinded, it is "still desirable and often possible to blind the assessor or obtain an objective source of data for evaluation of outcomes."[42]
Analysis of data
The types of statistical methods used in RCTs depend on the characteristics of the data and include:
- For dichotomous (binary) outcome data, logistic regression (e.g., to predict sustained virological response after receipt of peginterferon alfa-2a for hepatitis C[63]) and other methods can be used.
- For continuous outcome data, analysis of covariance (e.g., for changes in blood lipid levels after receipt of atorvastatin after acute coronary syndrome[64]) tests the effects of predictor variables.
- For time-to-event outcome data that may be censored, survival analysis (e.g., Kaplan–Meier estimators and Cox proportional hazards models for time to coronary heart disease after receipt of hormone replacement therapy in menopause[65]) is appropriate.
Regardless of the statistical methods used, important considerations in the analysis of RCT data include:
- Whether an RCT should be stopped early due to interim results. For example, RCTs may be stopped early if an intervention produces "larger than expected benefit or harm", or if "investigators find evidence of no important difference between experimental and control interventions."[4]
- The extent to which the groups can be analyzed exactly as they existed upon randomization (i.e., whether a so-called "intention-to-treat analysis" is used). A "pure" intention-to-treat analysis is "possible only when complete outcome data are available" for all randomized subjects;[66] when some outcome data are missing, options include analyzing only cases with known outcomes and using imputed data.[4] Nevertheless, the more that analyses can include all participants in the groups to which they were randomized, the less bias that an RCT will be subject to.[4]
- Whether subgroup analysis should be performed. These are "often discouraged" because multiple comparisons may produce false positive findings that cannot be confirmed by other studies.[4]
Reporting of results
The CONSORT 2010 Statement is "an evidence-based, minimum set of recommendations for reporting RCTs."[67] The CONSORT 2010 checklist contains 25 items (many with sub-items) focusing on "individually randomised, two group, parallel trials" which are the most common type of RCT.[1]
For other RCT study designs, "CONSORT extensions" have been published, some examples are:
- Consort 2010 Statement: Extension to Cluster Randomised Trials[68]
- Consort 2010 Statement: Non-Pharmacologic Treatment Interventions[69][70]
Relative importance and observational studies
Two studies published in The New England Journal of Medicine in 2000 found that observational studies and RCTs overall produced similar results.[71][72] The authors of the 2000 findings questioned the belief that "observational studies should not be used for defining evidence-based medical care" and that RCTs' results are "evidence of the highest grade."[71][72] However, a 2001 study published in Journal of the American Medical Association concluded that "discrepancies beyond chance do occur and differences in estimated magnitude of treatment effect are very common" between observational studies and RCTs.[73]
Two other lines of reasoning question RCTs' contribution to scientific knowledge beyond other types of studies:
- If study designs are ranked by their potential for new discoveries, then anecdotal evidence would be at the top of the list, followed by observational studies, followed by RCTs.[74]
- RCTs may be unnecessary for treatments that have dramatic and rapid effects relative to the expected stable or progressively worse natural course of the condition treated.[75][76] One example is combination chemotherapy including cisplatin for metastatic testicular cancer, which increased the cure rate from 5% to 60% in a 1977 non-randomized study.[76][77]
Interpretation of statistical results
Like all statistical methods, RCTs are subject to both type I ("false positive") and type II ("false negative") statistical errors. Regarding Type I errors, a typical RCT will use 0.05 (i.e., 1 in 20) as the probability that the RCT will falsely find two equally effective treatments significantly different.[78] Regarding Type II errors, despite the publication of a 1978 paper noting that the sample sizes of many "negative" RCTs were too small to make definitive conclusions about the negative results,[79] by 2005-2006 a sizeable proportion of RCTs still had inaccurate or incompletely reported sample size calculations.[80]
Peer review
Peer review of results is an important part of the scientific method. Reviewers examine the study results for potential problems with design that could lead to unreliable results (for example by creating a systematic bias), evaluate the study in the context of related studies and other evidence, and evaluate whether the study can be reasonably considered to have proven its conclusions. To underscore the need for peer review and the danger of over-generalizing conclusions, two Boston-area medical researchers performed a randomized controlled trial in which they randomly assigned either a parachute or an empty backpack to 23 volunteers who jumped from either a biplane or a helicopter. The study was able to accurately report that parachutes fail to reduce injury compared to empty backpacks. The key context that limited the general applicability of this conclusion was that the aircraft were parked on the ground, and participants had only jumped about two feet.[81]
Advantages
RCTs are considered to be the most reliable form of scientific evidence in the hierarchy of evidence that influences healthcare policy and practice because RCTs reduce spurious causality and bias. Results of RCTs may be combined in systematic reviews which are increasingly being used in the conduct of evidence-based practice. Some examples of scientific organizations' considering RCTs or systematic reviews of RCTs to be the highest-quality evidence available are:
- As of 1998, the National Health and Medical Research Council of Australia designated "Level I" evidence as that "obtained from a systematic review of all relevant randomised controlled trials" and "Level II" evidence as that "obtained from at least one properly designed randomised controlled trial."[82]
- Since at least 2001, in making clinical practice guideline recommendations the United States Preventive Services Task Force has considered both a study's design and its internal validity as indicators of its quality.[83] It has recognized "evidence obtained from at least one properly randomized controlled trial" with good internal validity (i.e., a rating of "I-good") as the highest quality evidence available to it.[83]
- The GRADE Working Group concluded in 2008 that "randomised trials without important limitations constitute high quality evidence."[84]
- For issues involving "Therapy/Prevention, Aetiology/Harm", the Oxford Centre for Evidence-based Medicine as of 2011 defined "Level 1a" evidence as a systematic review of RCTs that are consistent with each other, and "Level 1b" evidence as an "individual RCT (with narrow Confidence Interval)."[85]
Notable RCTs with unexpected results that contributed to changes in clinical practice include:
- After Food and Drug Administration approval, the antiarrhythmic agents flecainide and encainide came to market in 1986 and 1987 respectively.[86] The non-randomized studies concerning the drugs were characterized as "glowing",[87] and their sales increased to a combined total of approximately 165,000 prescriptions per month in early 1989.[86] In that year, however, a preliminary report of an RCT concluded that the two drugs increased mortality.[88] Sales of the drugs then decreased.[86]
- Prior to 2002, based on observational studies, it was routine for physicians to prescribe hormone replacement therapy for post-menopausal women to prevent myocardial infarction.[87] In 2002 and 2004, however, published RCTs from the Women's Health Initiative claimed that women taking hormone replacement therapy with estrogen plus progestin had a higher rate of myocardial infarctions than women on a placebo, and that estrogen-only hormone replacement therapy caused no reduction in the incidence of coronary heart disease.[65][89] Possible explanations for the discrepancy between the observational studies and the RCTs involved differences in methodology, in the hormone regimens used, and in the populations studied.[90][91] The use of hormone replacement therapy decreased after publication of the RCTs.[92]
Disadvantages
Many papers discuss the disadvantages of RCTs.[75][93][94] Among the most frequently cited drawbacks are:
Time and costs
RCTs can be expensive;[94] one study found 28 Phase III RCTs funded by the National Institute of Neurological Disorders and Stroke prior to 2000 with a total cost of US$335 million,[95] for a mean cost of US$12 million per RCT. Nevertheless, the return on investment of RCTs may be high, in that the same study projected that the 28 RCTs produced a "net benefit to society at 10-years" of 46 times the cost of the trials program, based on evaluating a quality-adjusted life year as equal to the prevailing mean per capita gross domestic product.[95]
The conduct of an RCT takes several years until being published; thus, data is restricted from the medical community for long years and may be of less relevance at time of publication.[96]
It is costly to maintain RCTs for the years or decades that would be ideal for evaluating some interventions.[75][94]
Interventions to prevent events that occur only infrequently (e.g., sudden infant death syndrome) and uncommon adverse outcomes (e.g., a rare side effect of a drug) would require RCTs with extremely large sample sizes and may, therefore, best be assessed by observational studies.[75]
Due to the costs of running RCTs, these usually only inspect one variable or very few variables, rarely reflecting the full picture of a complicated medical situation; whereas the case report, for example, can detail many aspects of the patient's medical situation (e.g. patient history, physical examination, diagnosis, psychosocial aspects, follow up).[96]
Conflict of interest dangers
A 2011 study done to disclose possible conflicts of interests in underlying research studies used for medical meta-analyses reviewed 29 meta-analyses and found that conflicts of interests in the studies underlying the meta-analyses were rarely disclosed. The 29 meta-analyses included 11 from general medicine journals; 15 from specialty medicine journals, and 3 from the Cochrane Database of Systematic Reviews. The 29 meta-analyses reviewed an aggregate of 509 randomized controlled trials (RCTs). Of these, 318 RCTs reported funding sources with 219 (69%) industry funded. 132 of the 509 RCTs reported author conflict of interest disclosures, with 91 studies (69%) disclosing industry financial ties with one or more authors. The information was, however, seldom reflected in the meta-analyses. Only two (7%) reported RCT funding sources and none reported RCT author-industry ties. The authors concluded "without acknowledgment of COI due to industry funding or author industry financial ties from RCTs included in meta-analyses, readers' understanding and appraisal of the evidence from the meta-analysis may be compromised."[97]
Some RCTs are fully or partly funded by the health care industry (e.g., the pharmaceutical industry) as opposed to government, nonprofit, or other sources. A systematic review published in 2003 found four 1986–2002 articles comparing industry-sponsored and nonindustry-sponsored RCTs, and in all the articles there was a correlation of industry sponsorship and positive study outcome.[98] A 2004 study of 1999–2001 RCTs published in leading medical and surgical journals determined that industry-funded RCTs "are more likely to be associated with statistically significant pro-industry findings."[99] These results have been mirrored in trials in surgery, where although industry funding did not affect the rate of trial discontinuation it was however associated with a lower odds of publication for completed trials.[100] One possible reason for the pro-industry results in industry-funded published RCTs is publication bias.[99] Other authors have cited the differing goals of academic and industry sponsored research as contributing to the difference. Commercial sponsors may be more focused on performing trials of drugs that have already shown promise in early stage trials, and on replicating previous positive results to fulfill regulatory requirements for drug approval.[101]
Ethics
If a disruptive innovation in medical technology is developed, it may be difficult to test this ethically in an RCT if it becomes "obvious" that the control subjects have poorer outcomes—either due to other foregoing testing, or within the initial phase of the RCT itself. Ethically it may be necessary to abort the RCT prematurely, and getting ethics approval (and patient agreement) to withhold the innovation from the control group in future RCT's may not be feasible.
Historical control trials (HCT) exploit the data of previous RCTs to reduce the sample size; however, these approaches are controversial in the scientific community and must be handled with care.[102]
In social science
Due to the recent emergence of RCTs in social science, the use of RCTs in social sciences is a contested issue. Some writers from a medical or health background have argued that existing research in a range of social science disciplines lacks rigour, and should be improved by greater use of randomized control trials.
Transport science
Researchers in transport science argue that public spending on programmes such as school travel plans could not be justified unless their efficacy is demonstrated by randomized controlled trials.[103] Graham-Rowe and colleagues[104] reviewed 77 evaluations of transport interventions found in the literature, categorising them into 5 "quality levels". They concluded that most of the studies were of low quality and advocated the use of randomized controlled trials wherever possible in future transport research.
Dr. Steve Melia[105] took issue with these conclusions, arguing that claims about the advantages of RCTs, in establishing causality and avoiding bias, have been exaggerated. He proposed the following eight criteria for the use of RCTs in contexts where interventions must change human behaviour to be effective:
The intervention:
- Has not been applied to all members of a unique group of people (e.g. the population of a whole country, all employees of a unique organisation etc.)
- Is applied in a context or setting similar to that which applies to the control group
- Can be isolated from other activities—and the purpose of the study is to assess this isolated effect
- Has a short timescale between its implementation and maturity of its effects
And the causal mechanisms:
- Are either known to the researchers, or else all possible alternatives can be tested
- Do not involve significant feedback mechanisms between the intervention group and external environments
- Have a stable and predictable relationship to exogenous factors
- Would act in the same way if the control group and intervention group were reversed
Criminology
A 2005 review found 83 randomized experiments in criminology published in 1982–2004, compared with only 35 published in 1957–1981.[106] The authors classified the studies they found into five categories: "policing", "prevention", "corrections", "court", and "community".[106] Focusing only on offending behavior programs, Hollin (2008) argued that RCTs may be difficult to implement (e.g., if an RCT required "passing sentences that would randomly assign offenders to programmes") and therefore that experiments with quasi-experimental design are still necessary.[107]
Education
RCTs have been used in evaluating a number of educational interventions. Between 1980 and 2016, over 1,000 reports of RCTs have been published.[108] For example, a 2009 study randomized 260 elementary school teachers' classrooms to receive or not receive a program of behavioral screening, classroom intervention, and parent training, and then measured the behavioral and academic performance of their students.[109] Another 2009 study randomized classrooms for 678 first-grade children to receive a classroom-centered intervention, a parent-centered intervention, or no intervention, and then followed their academic outcomes through age 19.[110]
Criticism
A 2017 review of the 10 most cited randomised controlled trials noted poor distribution of background traits, difficulties with blinding, and discussed other assumptions and biases inherent in randomised controlled trials. These include the "unique time period assessment bias", the "background traits remain constant assumption", the "average treatment effects limitation", the "simple treatment at the individual level limitation", the "all preconditions are fully met assumption", the "quantitative variable limitation" and the "placebo only or conventional treatment only limitation".[111]
See also
References
- Schulz KF, Altman DG, ((Moher D; for the CONSORT Group)) (2010). "CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials". Br Med J. 340: c332. doi:10.1136/bmj.c332. PMC 2844940. PMID 20332509.CS1 maint: multiple names: authors list (link)
- Chalmers TC, Smith H Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A (1981). "A method for assessing the quality of a randomized control trial". Controlled Clinical Trials. 2 (1): 31–49. doi:10.1016/0197-2456(81)90056-8. PMID 7261638.
- "Randomised controlled trial". National Institute for Health and Care Excellence, London, UK. 2019. Retrieved 3 June 2019.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG (2010). "CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials". Br Med J. 340: c869. doi:10.1136/bmj.c869. PMC 2844943. PMID 20332511.
- Hannan EL (June 2008). "Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations". JACC. Cardiovascular Interventions. 1 (3): 211–7. doi:10.1016/j.jcin.2008.01.008. PMID 19463302.
- Ranjith G (2005). "Interferon-α-induced depression: when a randomized trial is not a randomized controlled trial". Psychother Psychosom. 74 (6): 387, author reply 387–8. doi:10.1159/000087787. PMID 16244516. S2CID 143644933.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1976). "Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design". Br J Cancer. 34 (6): 585–612. doi:10.1038/bjc.1976.220. PMC 2025229. PMID 795448.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977). "Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples". Br J Cancer. 35 (1): 1–39. doi:10.1038/bjc.1977.1. PMC 2025310. PMID 831755.
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H (2004). "Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial". Lancet. 364 (9429): 141–8. doi:10.1016/S0140-6736(04)16626-9. PMID 15246726. S2CID 24361586.
- Dunn PM (January 1997). "James Lind (1716-94) of Edinburgh and the treatment of scurvy". Arch. Dis. Child. Fetal Neonatal Ed. 76 (1): F64–5. doi:10.1136/fn.76.1.f64. PMC 1720613. PMID 9059193.
- Charles Sanders Peirce and Joseph Jastrow (1885). "On Small Differences in Sensation". Memoirs of the National Academy of Sciences. 3: 73–83. http://psychclassics.yorku.ca/Peirce/small-diffs.htm
- Hacking, Ian (September 1988). "Telepathy: Origins of Randomization in Experimental Design". Isis. A Special Issue on Artifact and Experiment. 79 (3): 427–451. doi:10.1086/354775. JSTOR 234674. MR 1013489. S2CID 52201011.
- Stephen M. Stigler (November 1992). "A Historical View of Statistical Concepts in Psychology and Educational Research". American Journal of Education. 101 (1): 60–70. doi:10.1086/444032. S2CID 143685203.
- Trudy Dehue (December 1997). "Deception, Efficiency, and Random Groups: Psychology and the Gradual Origination of the Random Group Design" (PDF). Isis. 88 (4): 653–673. doi:10.1086/383850. PMID 9519574. S2CID 23526321.
- Neyman, Jerzy. 1923 [1990]. "On the Application of Probability Theory to AgriculturalExperiments. Essay on Principles. Section 9." Statistical Science 5 (4): 465–472. Trans. Dorota M. Dabrowska and Terence P. Speed.
-
According to Conniffe (1991, p. 87),
Ronald A. Fisher was "interested in application and in the popularization of statistical methods and his early book Statistical Methods for Research Workers, published in 1925, went through many editions and motivated and influenced the practical use of statistics in many fields of study. His Design of Experiments (1935) [promoted] statistical technique and application. In that book he emphasized examples and how to design experiments systematically from a statistical point of view. The mathematical justification of the methods described was not stressed and, indeed, proofs were often barely sketched or omitted altogether ..., a fact which led H. B. Mann to fill the gaps with a rigorous mathematical treatment in his well known treatise, Mann (1949)."
Page 87: Conniffe, Denis (1990–1991). "R. A. Fisher and the development of statistics—a view in his centenary year". Journal of the Statistical and Social Inquiry Society of Ireland. XXVI (3). Dublin: Statistical and Social Inquiry Society of Ireland. pp. 55–108. ISSN 0081-4776.Mann, H. B. (1949). Analysis and design of experiments: Analysis of variance and analysis of variance designs. New York, N. Y.: Dover Publications, Inc. pp. x+195. MR 0032177.
- Streptomycin in Tuberculosis Trials Committee (1948). "Streptomycin treatment of pulmonary tuberculosis. A Medical Research Council investigation". Br Med J. 2 (4582): 769–82. doi:10.1136/bmj.2.4582.769. PMC 2091872. PMID 18890300.
- Brown D (1998-11-02). "Landmark study made research resistant to bias". Washington Post.
- Shikata S, Nakayama T, Noguchi Y, Taji Y, Yamagishi H (2006). "Comparison of effects in randomized controlled trials with observational studies in digestive surgery". Ann Surg. 244 (5): 668–76. doi:10.1097/01.sla.0000225356.04304.bc. PMC 1856609. PMID 17060757.
- Stolberg HO, Norman G, Trop I (2004). "Randomized controlled trials". Am J Roentgenol. 183 (6): 1539–44. doi:10.2214/ajr.183.6.01831539. PMID 15547188.
- Georgina Ferry (2 November 2020). "Peter Sleight Obituary". The Guardian. Retrieved 3 November 2020.
- Meldrum ML (2000). "A brief history of the randomized controlled trial. From oranges and lemons to the gold standard". Hematol Oncol Clin North Am. 14 (4): 745–60, vii. doi:10.1016/S0889-8588(05)70309-9. PMID 10949771.
- Freedman B (1987). "Equipoise and the ethics of clinical research". N Engl J Med. 317 (3): 141–5. doi:10.1056/NEJM198707163170304. PMID 3600702.
- Gifford F (1995). "Community-equipoise and the ethics of randomized clinical trials". Bioethics. 9 (2): 127–48. doi:10.1111/j.1467-8519.1995.tb00306.x. PMID 11653056.
- Edwards SJ, Lilford RJ, Hewison J (1998). "The ethics of randomised controlled trials from the perspectives of patients, the public, and healthcare professionals". Br Med J. 317 (7167): 1209–12. doi:10.1136/bmj.317.7167.1209. PMC 1114158. PMID 9794861.
- Zelen M (1979). "A new design for randomized clinical trials". N Engl J Med. 300 (22): 1242–5. doi:10.1056/NEJM197905313002203. PMID 431682.
- Torgerson DJ, Roland M (1998). "What is Zelen's design?". Br Med J. 316 (7131): 606. doi:10.1136/bmj.316.7131.606. PMC 1112637. PMID 9518917.
- Appelbaum PS, Roth LH, Lidz C (1982). "The therapeutic misconception: informed consent in psychiatric research". Int J Law Psychiatry. 5 (3–4): 319–29. doi:10.1016/0160-2527(82)90026-7. PMID 6135666.
- Henderson GE, Churchill LR, Davis AM, Easter MM, Grady C, Joffe S, Kass N, King NM, Lidz CW, Miller FG, Nelson DK, Peppercorn J, Rothschild BB, Sankar P, Wilfond BS, Zimmer CR (2007). "Clinical trials and medical care: defining the therapeutic misconception". PLoS Med. 4 (11): e324. doi:10.1371/journal.pmed.0040324. PMC 2082641. PMID 18044980.
- Jain SL (2010). "The mortality effect: counting the dead in the cancer trial" (PDF). Public Culture. 21 (1): 89–117. doi:10.1215/08992363-2009-017. S2CID 143641293.
- De Angelis C, Drazen JM, Frizelle FA, et al. (September 2004). "Clinical trial registration: a statement from the International Committee of Medical Journal Editors". The New England Journal of Medicine. 351 (12): 1250–1. doi:10.1056/NEJMe048225. PMID 15356289.
- Law MR, Kawasumi Y, Morgan SG (2011). "Despite law, fewer than one in eight completed studies of drugs and biologics are reported on time on ClinicalTrials.gov". Health Aff (Millwood). 30 (12): 2338–45. doi:10.1377/hlthaff.2011.0172. PMID 22147862.
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P (2009). "Comparison of registered and published primary outcomes in randomized controlled trials". JAMA. 302 (9): 977–84. doi:10.1001/jama.2009.1242. PMID 19724045.
- Bhaumik, S (Mar 2013). "Editorial policies of MEDLINE indexed Indian journals on clinical trial registration". Indian Pediatr. 50 (3): 339–40. doi:10.1007/s13312-013-0092-2. PMID 23680610. S2CID 40317464.
- Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG (2010). "The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed". BMJ. 340: c723. doi:10.1136/bmj.c723. PMC 2844941. PMID 20332510.
- Kaiser, Joerg; Niesen, Willem; Probst, Pascal; Bruckner, Thomas; Doerr-Harim, Colette; Strobel, Oliver; Knebel, Phillip; Diener, Markus K.; Mihaljevic, André L.; Büchler, Markus W.; Hackert, Thilo (7 June 2019). "Abdominal drainage versus no drainage after distal pancreatectomy: study protocol for a randomized controlled trial". Trials. 20 (1): 332. doi:10.1186/s13063-019-3442-0. PMC 6555976. PMID 31174583.
- Farag, Sara M.; Mohammed, Manal O.; EL-Sobky, Tamer A.; ElKadery, Nadia A.; ElZohiery, Abeer K. (March 2020). "Botulinum Toxin A Injection in Treatment of Upper Limb Spasticity in Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials". JBJS Reviews. 8 (3): e0119. doi:10.2106/JBJS.RVW.19.00119. PMC 7161716. PMID 32224633.
- Jones, Byron; Kenward, Michael G. (2003). Design and Analysis of Cross-Over Trials (Second ed.). London: Chapman and Hall.
- Vonesh, Edward F.; Chinchilli, Vernon G. (1997). "Crossover Experiments". Linear and Nonlinear Models for the Analysis of Repeated Measurements. London: Chapman and Hall. pp. 111–202.
- Gall, Stefanie; Adams, Larissa; Joubert, Nandi; Ludyga, Sebastian; Müller, Ivan; Nqweniso, Siphesihle; Pühse, Uwe; du Randt, Rosa; Seelig, Harald; Smith, Danielle; Steinmann, Peter; Utzinger, Jürg; Walter, Cheryl; Gerber, Markus; van Wouwe, Jacobus P. (8 November 2018). "Effect of a 20-week physical activity intervention on selective attention and academic performance in children living in disadvantaged neighborhoods: A cluster randomized control trial". PLOS ONE. 13 (11): e0206908. Bibcode:2018PLoSO..1306908G. doi:10.1371/journal.pone.0206908. PMC 6224098. PMID 30408073.
- Gladstone, Melissa J.; Chandna, Jaya; Kandawasvika, Gwendoline; Ntozini, Robert; Majo, Florence D.; Tavengwa, Naume V.; Mbuya, Mduduzi N. N.; Mangwadu, Goldberg T.; Chigumira, Ancikaria; Chasokela, Cynthia M.; Moulton, Lawrence H.; Stoltzfus, Rebecca J.; Humphrey, Jean H.; Prendergast, Andrew J.; Tumwine, James K. (21 March 2019). "Independent and combined effects of improved water, sanitation, and hygiene (WASH) and improved complementary feeding on early neurodevelopment among children born to HIV-negative mothers in rural Zimbabwe: Substudy of a cluster-randomized trial". PLOS Medicine. 16 (3): e1002766. doi:10.1371/journal.pmed.1002766. PMC 6428259. PMID 30897095.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group (2008). "Improving the reporting of pragmatic trials: an extension of the CONSORT statement". BMJ. 337: a2390. doi:10.1136/bmj.a2390. PMC 3266844. PMID 19001484.CS1 maint: multiple names: authors list (link)
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group (2006). "Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement" (PDF). JAMA. 295 (10): 1152–60. doi:10.1001/jama.295.10.1152. PMID 16522836.CS1 maint: multiple names: authors list (link)
- Schulz KF, Grimes DA (2002). "Generation of allocation sequences in randomised trials: chance, not choice" (PDF). Lancet. 359 (9305): 515–9. doi:10.1016/S0140-6736(02)07683-3. PMID 11853818. S2CID 291300.
- Schulz KF, Grimes DA (2002). "Allocation concealment in randomised trials: defending against deciphering" (PDF). Lancet. 359 (9306): 614–8. doi:10.1016/S0140-6736(02)07750-4. PMID 11867132. S2CID 12902486.
- Howick J, Mebius A (2014). "In search of justification for the unpredictability paradox". Trials. 15: 480. doi:10.1186/1745-6215-15-480. PMC 4295227. PMID 25490908.
- Lachin JM (1988). "Statistical properties of randomization in clinical trials". Controlled Clinical Trials. 9 (4): 289–311. doi:10.1016/0197-2456(88)90045-1. PMID 3060315.
- Rosenberger, James. "STAT 503 - Design of Experiments". Pennsylvania State University. Retrieved 24 September 2012.
- Avins, A L (1998). ""Can unequal be more fair? Ethics, subject allocation, and randomized clinical trials"". J Med Ethics. 24 (6): 401–408. doi:10.1136/jme.24.6.401. PMC 479141. PMID 9873981.
- Buyse ME (1989). "Analysis of clinical trial outcomes: some comments on subgroup analyses". Controlled Clinical Trials. 10 (4 Suppl): 187S–194S. doi:10.1016/0197-2456(89)90057-3. PMID 2605967.
- Lachin JM, Matts JP, Wei LJ (1988). "Randomization in clinical trials: conclusions and recommendations" (PDF). Controlled Clinical Trials. 9 (4): 365–74. doi:10.1016/0197-2456(88)90049-9. hdl:2027.42/27041. PMID 3203526.
- Rosenberger WF, Lachin JM (1993). "The use of response-adaptive designs in clinical trials". Controlled Clinical Trials. 14 (6): 471–84. doi:10.1016/0197-2456(93)90028-C. PMID 8119063.
- Forder PM, Gebski VJ, Keech AC (2005). "Allocation concealment and blinding: when ignorance is bliss". Med J Aust. 182 (2): 87–9. doi:10.5694/j.1326-5377.2005.tb06584.x. PMID 15651970. S2CID 202149.
- Pildal J, Chan AW, Hróbjartsson A, Forfang E, Altman DG, Gøtzsche PC (2005). "Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study". BMJ. 330 (7499): 1049. doi:10.1136/bmj.38414.422650.8F. PMC 557221. PMID 15817527.
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA (2008). "Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study". BMJ. 336 (7644): 601–5. doi:10.1136/bmj.39465.451748.AD. PMC 2267990. PMID 18316340.
- Glennerster, Rachel; Kudzai Takavarasha (2013). ""Chapter 6"". Running randomized evaluations: a practical guide. Princeton: Princeton University Press. ISBN 9780691159249.
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, Bhandari M, Guyatt GH (2001). "Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials". J Am Med Assoc. 285 (15): 2000–3. doi:10.1001/jama.285.15.2000. PMID 11308438.
- Haahr MT, Hróbjartsson A (2006). "Who is blinded in randomized clinical trials? A study of 200 trials and a survey of authors". Clin Trials. 3 (4): 360–5. doi:10.1177/1740774506069153. PMID 17060210. S2CID 23818514.
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. (2007). "The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial". Lancet. 369 (9566): 1016–26. doi:10.1016/S0140-6736(07)60461-9. PMC 2039891. PMID 17382828.
- Chan R, Hemeryck L, O'Regan M, Clancy L, Feely J (1995). "Oral versus intravenous antibiotics for community acquired lower respiratory tract infection in a general hospital: open, randomised controlled trial". BMJ. 310 (6991): 1360–2. doi:10.1136/bmj.310.6991.1360. PMC 2549744. PMID 7787537.
- Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group (2008). "Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial" (PDF). Lancet. 372 (9636): 392–7. doi:10.1016/S0140-6736(08)61159-9. hdl:2115/34681. PMID 18675689. S2CID 13741892.CS1 maint: multiple names: authors list (link)
- Noseworthy JH, Ebers GC, Vandervoort MK, Farquhar RE, Yetisir E, Roberts R (1994). "The impact of blinding on the results of a randomized, placebo-controlled multiple sclerosis clinical trial". Neurology. 44 (1): 16–20. doi:10.1212/wnl.44.1.16. PMID 8290055. S2CID 2663997.
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK (2001). "Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial". Lancet. 358 (9286): 958–65. doi:10.1016/S0140-6736(01)06102-5. PMID 11583749. S2CID 14583372.
- Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators (2001). "Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial". J Am Med Assoc. 285 (13): 1711–8. doi:10.1001/jama.285.13.1711. PMID 11277825.CS1 maint: multiple names: authors list (link)
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators (2002). "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial" (PDF). J Am Med Assoc. 288 (3): 321–33. doi:10.1001/jama.288.3.321. PMID 12117397. S2CID 20149703.CS1 maint: multiple names: authors list (link)
- Hollis S, Campbell F (1999). "What is meant by intention to treat analysis? Survey of published randomised controlled trials". Br Med J. 319 (7211): 670–4. doi:10.1136/bmj.319.7211.670. PMC 28218. PMID 10480822.
- CONSORT Group. "Welcome to the CONSORT statement Website". Retrieved 2010-03-29.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG (2012). "Consort 2010 statement: extension to cluster randomised trials". BMJ. 345: e5661. doi:10.1136/bmj.e5661. PMID 22951546.
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P (2008). "Extending the CONSORT Statement to randomized trials of nonpharmacologic treatment: explanation and elaboration". Annals of Internal Medicine. 148 (4): 295–309. doi:10.7326/0003-4819-148-4-200802190-00008. PMID 18283207.
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P (2008). "Methods and Processes of the CONSORT Group: Example of an Extension for Trials Assessing Nonpharmacologic Treatments". Annals of Internal Medicine. 148 (4): W60–6. doi:10.7326/0003-4819-148-4-200802190-00008-w1. PMID 18283201.
- Benson K, Hartz AJ (2000). "A comparison of observational studies and randomized, controlled trials". N Engl J Med. 342 (25): 1878–86. doi:10.1056/NEJM200006223422506. PMID 10861324.
- Concato J, Shah N, Horwitz RI (2000). "Randomized, controlled trials, observational studies, and the hierarchy of research designs". N Engl J Med. 342 (25): 1887–92. doi:10.1056/NEJM200006223422507. PMC 1557642. PMID 10861325.
- Ioannidis JP, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, Contopoulos-Ioannidis DG, Lau J (2001). "Comparison of evidence of treatment effects in randomized and nonrandomized studies". J Am Med Assoc. 286 (7): 821–30. CiteSeerX 10.1.1.590.2854. doi:10.1001/jama.286.7.821. PMID 11497536.
- Vandenbroucke JP (2008). "Observational research, randomised trials, and two views of medical science". PLoS Med. 5 (3): e67. doi:10.1371/journal.pmed.0050067. PMC 2265762. PMID 18336067.
- Black N (1996). "Why we need observational studies to evaluate the effectiveness of health care". BMJ. 312 (7040): 1215–8. doi:10.1136/bmj.312.7040.1215. PMC 2350940. PMID 8634569.
- Glasziou P, Chalmers I, Rawlins M, McCulloch P (2007). "When are randomised trials unnecessary? Picking signal from noise". Br Med J. 334 (7589): 349–51. doi:10.1136/bmj.39070.527986.68. PMC 1800999. PMID 17303884.
- Einhorn LH (2002). "Curing metastatic testicular cancer". Proc Natl Acad Sci U S A. 99 (7): 4592–5. doi:10.1073/pnas.072067999. PMC 123692. PMID 11904381.
- Wittes J (2002). "Sample size calculations for randomized controlled trials". Epidemiol Rev. 24 (1): 39–53. doi:10.1093/epirev/24.1.39. PMID 12119854.
- Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR (1978). "The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials". N Engl J Med. 299 (13): 690–4. doi:10.1056/NEJM197809282991304. PMID 355881.
- Charles P, Giraudeau B, Dechartres A, Baron G, Ravaud P (2009-05-12). "Reporting of sample size calculation in randomised controlled trials: review". Br Med J. 338: b1732. doi:10.1136/bmj.b1732. PMC 2680945. PMID 19435763.
- Richard Harris (22 Dec 2018). "Researchers Show Parachutes Don't Work, But There's A Catch".
- National Health and Medical Research Council (1998-11-16). A guide to the development, implementation and evaluation of clinical practice guidelines (PDF). Canberra: Commonwealth of Australia. p. 56. ISBN 978-1-86496-048-8. Retrieved 2010-03-28.
- Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D; Methods Work Group, Third US Preventive Services Task Force (2001). "Current methods of the US Preventive Services Task Force: a review of the process" (PDF). Am J Prev Med. 20 (3 Suppl): 21–35. doi:10.1016/S0749-3797(01)00261-6. PMID 11306229.CS1 maint: multiple names: authors list (link)
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group (2008). "What is "quality of evidence" and why is it important to clinicians?". BMJ. 336 (7651): 995–8. doi:10.1136/bmj.39490.551019.BE. PMC 2364804. PMID 18456631.CS1 maint: multiple names: authors list (link)
- Oxford Centre for Evidence-based Medicine (2011-09-16). "Levels of evidence". Retrieved 2012-02-15.
- Anderson JL, Pratt CM, Waldo AL, Karagounis LA (1997). "Impact of the Food and Drug Administration approval of flecainide and encainide on coronary artery disease mortality: putting "Deadly Medicine" to the test". Am J Cardiol. 79 (1): 43–7. doi:10.1016/S0002-9149(96)00673-X. PMID 9024734.
- Rubin R (2006-10-16). "In medicine, evidence can be confusing - deluged with studies, doctors try to sort out what works, what doesn't". USA Today. Retrieved 2010-03-22.
- Cardiac Arrhythmia Suppression Trial (CAST) Investigators (1989). "Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators". N Engl J Med. 321 (6): 406–12. doi:10.1056/NEJM198908103210629. PMID 2473403.
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. (2004). "Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial". JAMA. 291 (14): 1701–12. doi:10.1001/jama.291.14.1701. PMID 15082697.
- Grodstein F, Clarkson TB, Manson JE (2003). "Understanding the divergent data on postmenopausal hormone therapy". N Engl J Med. 348 (7): 645–50. doi:10.1056/NEJMsb022365. PMID 12584376.
- Vandenbroucke JP (2009). "The HRT controversy: observational studies and RCTs fall in line". Lancet. 373 (9671): 1233–5. doi:10.1016/S0140-6736(09)60708-X. PMID 19362661. S2CID 44991220.
- Hsu A, Card A, Lin SX, Mota S, Carrasquillo O, Moran A (2009). "Changes in postmenopausal hormone replacement therapy use among women with high cardiovascular risk". Am J Public Health. 99 (12): 2184–7. doi:10.2105/AJPH.2009.159889. PMC 2775780. PMID 19833984.
- Bell, S.H., & Peck, L.R. (2012). "Obstacles to and limitations of social experiments: 15 false alarms". Abt Thought Leadership Paper Series.CS1 maint: multiple names: authors list (link)
- Sanson-Fisher RW, Bonevski B, Green LW, D'Este C (2007). "Limitations of the randomized controlled trial in evaluating population-based health interventions". Am J Prev Med. 33 (2): 155–61. doi:10.1016/j.amepre.2007.04.007. PMID 17673104.
- Johnston SC, Rootenberg JD, Katrak S, Smith WS, Elkins JS (2006). "Effect of a US National Institutes of Health programme of clinical trials on public health and costs" (PDF). Lancet. 367 (9519): 1319–27. doi:10.1016/S0140-6736(06)68578-4. PMID 16631910. S2CID 41035177.
- Yitschaky O, Yitschaky M, Zadik Y (May 2011). "Case report on trial: Do you, Doctor, swear to tell the truth, the whole truth and nothing but the truth?" (PDF). J Med Case Rep. 5 (1): 179. doi:10.1186/1752-1947-5-179. PMC 3113995. PMID 21569508.
- "How Well Do Meta-Analyses Disclose Conflicts of Interests in Underlying Research Studies | The Cochrane Collaboration". Cochrane.org. Retrieved 2011-08-19.
- Bekelman JE, Li Y, Gross CP (2003). "Scope and impact of financial conflicts of interest in biomedical research: a systematic review". J Am Med Assoc. 289 (4): 454–65. doi:10.1001/jama.289.4.454. PMID 12533125.
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ (2004). "Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials". Can Med Assoc J. 170 (4): 477–80. PMC 332713. PMID 14970094.
- Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A (2014). "Discontinuation and non-publication of surgical randomised controlled trials: observational study". BMJ. 349: g6870. doi:10.1136/bmj.g6870. PMC 4260649. PMID 25491195.
- Ridker PM, Torres J (2006). "Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005". JAMA. 295 (19): 2270–4. doi:10.1001/jama.295.19.2270. PMID 16705108.
- Song Zhang; Jing Cao; Ahn, C. (23 June 2010). "Calculating sample size in trials using historical controls". Clinical Trials: Journal of the Society for Clinical Trials. 7 (4): 343–353. doi:10.1177/1740774510373629. PMC 3085081. PMID 20573638.
- Rowland, D., DiGuiseppi, C., Gross, M., Afolabi, E. and Roberts, I. (2003). "Randomised controlled trial of site specific advice on school travel patterns". Archives of Disease in Childhood. 88 (1): 8–11. doi:10.1136/adc.88.1.8. PMC 1719287. PMID 12495948.CS1 maint: multiple names: authors list (link)
- Graham-Rowe, E., Skippon, S., Gardner, B. and Abraham, C. (2011). "Can we reduce car use and, if so, how? A review of available evidence". Transportation Research Part A: Policy and Practice. 44 (5): 401–418. doi:10.1016/j.tra.2011.02.001.CS1 maint: multiple names: authors list (link)
- Melia(2011) Do Randomised Control Trials Offer a Solution to ’low Quality’ Transport Research? Bristol: University of the West of England]
- Farrington DP, Welsh BC (2005). "Randomized experiments in criminology: What have we learned in the last two decades?". Journal of Experimental Criminology. 1 (1): 9–38. doi:10.1007/s11292-004-6460-0. S2CID 145758503.
- Hollin CR (2008). "Evaluating offending behaviour programmes: does only randomization glister?". Criminology and Criminal Justice. 8 (1): 89–106. doi:10.1177/1748895807085871. S2CID 141222135.
- Connolly, Paul; Keenan, Ciara; Urbanska, Karolina (2018-07-09). "The trials of evidence-based practice in education: a systematic review of randomised controlled trials in education research 1980–2016". Educational Research. 60 (3): 276–291. doi:10.1080/00131881.2018.1493353. ISSN 0013-1881.
- Walker HM, Seeley JR, Small J, Severson HH, Graham BA, Feil EG, Serna L, Golly AM, Forness SR (2009). "A randomized controlled trial of the First Step to Success early intervention. Demonstration of program efficacy outcomes in a diverse, urban school district". Journal of Emotional and Behavioral Disorders. 17 (4): 197–212. doi:10.1177/1063426609341645. S2CID 144571336.
- Bradshaw CP, Zmuda JH, Kellam SG, Ialongo NS (2009). "Longitudinal impact of two universal preventive interventions in first grade on educational outcomes in high school". Journal of Educational Psychology. 101 (4): 926–937. doi:10.1037/a0016586. PMC 3678772. PMID 23766545.
- Krauss, Alexander (2018-05-19). "Why all randomised controlled trials produce biased results". Annals of Medicine. 50 (4): 312–322. doi:10.1080/07853890.2018.1453233. ISSN 0785-3890. PMID 29616838.
External links
- Bland M. Directory of randomisation software and services. University of York, 2008 March 19.
- Evans I, Thornton H, Chalmers I. Testing treatments: better research for better health care. London: Pinter & Martin, 2010. ISBN 978-1-905177-35-6.
- Gelband H. The impact of randomized clinical trials on health policy and medical practice: background paper. Washington, DC: U.S. Congress, Office of Technology Assessment, 1983. (Report OTA-BP-H-22.)
- REFLECT (Reporting guidElines For randomized controLled trials for livEstoCk and food safeTy) Statement
- Wathen JK, Cook JD. Power and bias in adaptively randomized clinical trials. M. D. Anderson Cancer Center, University of Texas, 2006 July 12.
