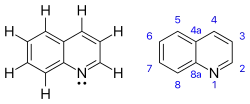
Quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents.[4] Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified.[5][6] 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Quinoline[2] | |||
Systematic IUPAC name
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.865 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Quinolines | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2656 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H7N | |||
| Molar mass | 129.16 g/mol | ||
| Appearance | Colorless oily liquid | ||
| Density | 1.093 g/mL | ||
| Melting point | −15 °C (5 °F; 258 K) | ||
| Boiling point | 237 °C (459 °F; 510 K) , 760 mm Hg; 108–110 °C (226–230 °F), 11 mm Hg | ||
| Slightly soluble | |||
| Solubility | Soluble in alcohol, ether, and carbon disulfide | ||
| Acidity (pKa) | 4.85 (conjugated acid)[3] | ||
| −86.0·10−6 cm3/mol | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
174.9 kJ·mol−1 | ||
| Hazards | |||
| R-phrases (outdated) | R21, R22 | ||
| S-phrases (outdated) | S26, S27, S28, S29, S30, S31, S32, S33, S34, S35, S36 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 101 °C (214 °F; 374 K) | ||
| 400 °C (752 °F; 673 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
331 mg/kg | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Occurrence and isolation
Quinoline was first extracted from coal tar in 1834 by German chemist Friedlieb Ferdinand Runge;[4] he called quinoline leukol ("white oil" in Greek).[7] Coal tar remains the principal source of commercial quinoline.[8] In 1842, French chemist Charles Gerhardt obtained a compound by dry distilling quinine, strychnine, or cinchonine with potassium hydroxide;[4] he called the compound Chinoilin or Chinolein.[9] Runge's and Gephardt's compounds seemed to be distinct isomers because they reacted differently. However, the German chemist August Hoffmann eventually recognized that the differences in behaviors was due to the presence of contaminants and that the two compounds were actually identical.[10] The only report of quinoline as a natural product is from the Peruvian stick insect Oreophoetes peruana. They have a pair of thoracic glands from which they discharge a malodorous fluid containing quinoline when disturbed. (Eisner, T; Morgan, R.C.; Attygalle A.B., Smedley, S.R.; Herath, K.B., Meinwald, J. (1997) “Defensive Production of quinoline by a phasmid insect (Oreophoetes peruana) J. Exp. Biol. 200, 2493–2500).
Like other nitrogen heterocyclic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites. Owing to its relatively high solubility in water quinoline has significant potential for mobility in the environment, which may promote water contamination. Quinoline is readily degradable by certain microorganisms, such as Rhodococcus species Strain Q1, which was isolated from soil and paper mill sludge.[11]
Quinolines are present in small amounts in crude oil within the virgin diesel fraction. It can be removed by the process called hydrodenitrification.
Synthesis
Quinolines are often synthesized from simple anilines using a number of named reactions.
Going clockwise from top these are:
- Combes quinoline synthesis using anilines and β-diketones.
- Conrad-Limpach synthesis using anilines and β-ketoesters.
- Doebner reaction using anilines with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids
- Doebner-Miller reaction using anilines and α,β-unsaturated carbonyl compounds.
- Gould-Jacobs reaction starting from an aniline and ethyl ethoxymethylenemalonate
- Skraup synthesis using ferrous sulfate, glycerol, aniline, nitrobenzene, and sulfuric acid.
A number of other processes exist, which require specifically substituted anilines or related compounds:
- Camps quinoline synthesis using an o-acylaminoacetophenone and hydroxide
- Friedländer synthesis using 2-aminobenzaldehyde and acetaldehyde
- Knorr quinoline synthesis, using a β-ketoanilide and sulfuric acid
- Niementowski quinoline synthesis, using anthranilic acid and ketones
- Pfitzinger reaction using an isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids
- Povarov reaction using an aniline, a benzaldehyde and an activated alkene
Applications
Quinoline is used in the manufacture of dyes, the preparation of hydroxyquinoline sulfate and niacin. It is also used as a solvent for resins and terpenes.
Quinoline is mainly used as in the production of other specialty chemicals. Approximately 4 tonnes are produced annually according to a report published in 2005.[8] Its principal use is as a precursor to 8-hydroxyquinoline, which is a versatile chelating agent and precursor to pesticides. Its 2- and 4-methyl derivatives are precursors to cyanine dyes. Oxidation of quinoline affords quinolinic acid (pyridine-2,3-dicarboxylic acid), a precursor to the herbicide sold under the name "Assert".[8]
The reduction of quinoline with sodium borohydride in the presence of acetic acid is known to produce Kairoline A.[12] (C.f. Kairine)
Quinoline has several anti-malarial derivatives, including quinine, chloroquine, amodiaquine, and primaquine.
Quinolines are reduced to tetrahydroquinolines enantioselectively using several catalyst systems.[13][14]

See also
- Quinoline alkaloids
- 4-Aminoquinoline
- 8-Hydroxyquinoline
- Pyrroloquinoline quinone (PQQ), a redox cofactor and controversial nutritional supplement
- Quinazoline, an aza derivative of quinoline
- Quinine
- Similar simple aromatic rings
- Isoquinoline, an analog with the nitrogen atom in position 2
- Pyridine, an analog without the fused benzene ring
- Naphthalene, an analog with a carbon instead of the nitrogen
- Indole, an analog with only a five-membered nitrogen ring
References
- "QUINOLINE (BENZOPYRIDINE)". Chemicalland21.com. Retrieved 2012-06-14.
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 4, 211. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The name ‘quinoline’ is a retained name that is preferred to the alternative systematic fusion names ‘1-benzopyridine’ or ‘benzo[b]pyridine’.
- Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 22 (11th ed.). Cambridge University Press. p. 759.
- Shang, XF; Morris-Natschke, SL; Liu, YQ; Guo, X; Xu, XS; Goto, M; Li, JC; Yang, GZ; Lee, KH (May 2018). "Biologically active quinoline and quinazoline alkaloids part I." Medicinal Research Reviews. 38 (3): 775–828. doi:10.1002/med.21466. PMC 6421866. PMID 28902434.
- Shang, Xiao-Fei; Morris-Natschke, Susan L.; Yang, Guan-Zhou; Liu, Ying-Qian; Guo, Xiao; Xu, Xiao-Shan; Goto, Masuo; Li, Jun-Cai; Zhang, Ji-Yu; Lee, Kuo-Hsiung (September 2018). "Biologically active quinoline and quinazoline alkaloids part II". Medicinal Research Reviews. 38 (5): 1614–1660. doi:10.1002/med.21492. PMC 6105521. PMID 29485730.
- F. F. Runge (1834) "Ueber einige Produkte der Steinkohlendestillation" (On some products of coal distillation), Annalen der Physik und Chemie, 31 (5) : 65–78 ; see especially p. 68: "3. Leukol oder Weissöl" (3. White oil [in Greek] or white oil [in German]). From p. 68: "Diese dritte Basis habe ich Leukol oder Weissöl genannt, weil sie keine farbigen Reactionen zeigt." (This third base I've named leukol or white oil because it shows no color reactions.)
- Gerd Collin; Hartmut Höke. "Quinoline and Isoquinoline". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_465.
- Gerhardt, Ch. (1842) "Untersuchungen über die organischen Basen" (Investigations of organic bases), Annalen der Chemie und Pharmacie, 42 : 310-313. See also: (Editor) (1842) "Chinolein oder Chinoilin" (Quinoline or quinoilin), Annalen der Chemie und Pharmacie, 44 : 279-280.
- Initially, Hoffmann thought that Runge's Leukol and Gerhardt's Chinolein were distinct. (See: Hoffmann, August Wilhelm (1843) "Chemische Untersuchungen der organischen Basen im Steinkohlen-Theeröl" (Chemical investigations of organic bases in coal tar oil), Annalen der Chemie und Pharmacie, 47 : 37-87 ; see especially pp. 76-78.) However, after further purification of his Leukol sample, Hoffmann determined that the two were indeed identical. (See: (Editor) (1845) "Vorläufige Notiz über die Identität des Leukols und Chinolins" (Preliminary notice of the identity of leukol and quinoline), Annalen der Chemie und Pharmacie, 53 : 427-428.)
- O'Loughlin, Edward J.; Kehrmeyer, Staci R.; Sims, Gerald K. (1996). "Isolation, characterization, and substrate utilization of a quinoline-degrading bacterium". International Biodeterioration & Biodegradation. 38 (2): 107. doi:10.1016/S0964-8305(96)00032-7.
- GRIBBLE, Gordon W.; HEALD, Peter W. (1975). "Reactions of Sodium Borohydride in Acidic Media; III. Reduction and Alkylation of Quinoline and Isoquinoline with Carboxylic Acids". Synthesis. 1975 (10): 650–652. doi:10.1055/s-1975-23871. ISSN 0039-7881.
- Xu, L.; Lam, K. H.; Ji, J.; Wu, J.; Fan, Q.-H.; Lo, W.-H.; Chan, A. S. C. Chem. Commun. 2005, 1390.
- Reetz, M. T.; Li, X. Chem. Commun. 2006, 2159.
External links
| Wikimedia Commons has media related to Quinoline. |