Reductive amination
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.[1][2]
| Reductive amination | |
|---|---|
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000335 |
Reaction process
In this organic reaction, the amine first reacts with the carbonyl group to form a hemiaminal species, which subsequently loses one molecule of water in a reversible manner by alkylimino-de-oxo-bisubstitution, to form the imine. The equilibrium between aldehyde/ketone and imine can be shifted toward imine formation by removal of the formed water through physical or chemical means. This intermediate imine can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride). This method is sometimes called indirect reductive amination.
In a separate approach, imine formation and reduction can occur sequentially in one pot. This approach, known as direct reductive amination, employs reducing agents that are more reactive toward protonated imines than towards the ketone/aldehyde precursors. These hydride reagents also must tolerate moderately acidic conditions. Typical reagents that meet these criteria include sodium cyanoborohydride (NaBH3CN) and sodium triacetoxyborohydride (NaBH(OCOCH3)3).[3] This reaction can be performed in an aqueous environment, which casts doubt on the necessity of forming the imine.[4][5] Possibly the reaction proceeds via reduction of the hemiaminal species.[6]
Variations and related reactions
This reaction is related to the Eschweiler–Clarke reaction, in which amines are methylated to tertiary amines, the Leuckart–Wallach reaction,[7] or by other amine alkylation methods such as the Mannich reaction and Petasis reaction.
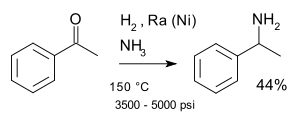
A classic named reaction is the Mignonac reaction (1921)[8] involving reaction of a ketone with ammonia over a nickel catalyst for example in a synthesis of 1-phenylethylamine starting from acetophenone:[9]
Nowadays, one-pot reductive amination fulfil by acid-metal catalysts that act as a hydride transfer. Much research study on this kind of reaction show high efficiency.[10]
In industry, tertiary amines such as triethylamine and diisopropylethylamine are formed directly from ketones with a gaseous mixture of ammonia and hydrogen and a suitable catalyst.
Biochemistry
A step in the biosynthesis of many α-amino acids is the reductive amination of an α-ketoacid, usually by a transaminase enzyme. The process is catalyzed by pyridoxamine phosphate, which is converted into pyridoxal phosphate after the reaction. The initial step entails formation of an imine, but the hydride equivalents are supplied by a reduced pyridine to give an aldimine, which hydrolyzes to the amine.[11] The sequence from keto-acid to amino acid can be summarized as follows:
- HO2CC(O)R → HO2CC(=NCH2–X)R → HO2CCH(N=CH–X)R → HO2CCH(NH2)R.
In popular culture
In the critically acclaimed drama Breaking Bad, main character Walter White uses the reductive amination reaction to produce his high purity methamphetamine, relying on 2-phenyl-propanone and methylamine. The 2-phenyl-propanone (otherwise known as phenylacetone or P2P) is produced from phenylacetic acid and acetic acid using a tube furnace and thorium dioxide (ThO2) as a catalyst.
See also
References
- Warren, Stuart & Wyatt, Paul (2008). Organic Synthesis : the disconnection approach (2nd ed.). Oxford: Wiley-Blackwell. p. 54. ISBN 978-0-470-71236-8.
- Afanasyev, Oleg I.; Kuchuk, Ekaterina; Usanov, Dmitry L.; Chusov, Denis (21 October 2019). "Reductive Amination in the Synthesis of Pharmaceuticals". Chemical Reviews. doi:10.1021/acs.chemrev.9b00383.
- Baxter, Ellen W.; Reitz, Allen B. (2004). "Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents". In Overman, Larry E. (ed.). Organic Reactions. pp. 1–714. doi:10.1002/0471264180.or059.01. ISBN 978-0-471-17655-8.
- Sato, Shinya; Sakamoto, Takeshi; Miyazawa, Etsuko; Kikugawa, Yasuo (2004). "One-pot reductive amination of aldehydes and ketones with α-picoline-borane in methanol, in water, and in neat conditions". Tetrahedron. 60 (36): 7899–906. doi:10.1016/j.tet.2004.06.045.
- Dunsmore, Colin J.; Carr, Reuben; Fleming, Toni; Turner, Nicholas J. (2006). "A Chemo-Enzymatic Route to Enantiomerically Pure Cyclic Tertiary Amines". Journal of the American Chemical Society. 128 (7): 2224–5. doi:10.1021/ja058536d. PMID 16478171.
- Tarasevich, Vladimir A; Kozlov, Nikolai G (1999). "Reductive amination of oxygen-containing organic compounds". Russian Chemical Reviews. 68 (1): 55–72. Bibcode:1999RuCRv..68...55T. doi:10.1070/RC1999v068n01ABEH000389.
- George, Frederick & Saunders, Bernard (1960). Practical Organic Chemistry, 4th Ed. London: Longman. p. 223. ISBN 9780582444072.
- Mignonac, Georges (1921). "Nouvelle méthode générale de préparation des amines à partir des aldéhydes ou des cétones" [New general method for preparation of amines from aldehydes or ketones]. Comptes rendus (in French). 172: 223.
- Robinson, John C.; Snyder, H. R. (1955). "α-Phenylethylamine". Organic Syntheses. doi:10.1002/0471264180.os023.27.; Collective Volume, 3, p. 717
- Kalbasi, Roozbeh Javad; Mazaheri, Omid (2015). "Synthesis and characterization of hierarchical ZSM-5 zeolite containing Ni nanoparticles for one-pot reductive amination of aldehydes with nitroarenes". Catalysis Communications. 69: 86–91. doi:10.1016/j.catcom.2015.05.016.
- Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
External links
- Current methods for reductive amination
- Industrial reductive amination at BASF