Forster–Decker method
The Forster–Decker method is a series of chemical reactions that transform a primary amine (1) ultimately to a secondary amine (6).[1][2] The first step is the formation of a Schiff base (3), followed by alkylation, and hydrolysis.
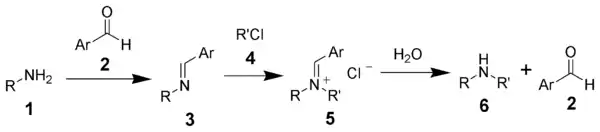
The Forster-Decker method
See also
References
- Forster, Martin Onslow (1899). "XCI.–Influence of substitution on specific rotation in the bornylamine series". Journal of the Chemical Society, Transactions. 75: 934–935. doi:10.1039/CT8997500934.
- Decker, H.; Becker, P. Ann. 1913, 395, 362.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
