Resorcinarene
A resorcinarene (also resorcarene or calix[4]resorcinarene) is a macrocycle, or a cyclic oligomer, based on the condensation of resorcinol (1,3-dihydroxybenzene) and an aldehyde. Resorcinarenes are a type of calixarene. Other types of resorcinarenes include the related pyrogallolarenes and octahydroxypyridines, derived from pyrogallol and 2,6-dihydroxypyridine, respectively.
Resorcinarenes interact with other molecules forming a host–guest complex.[1] Resorcinarenes and pyrogallolarenes self-assemble into larger supramolecular structures. Both in the crystalline state and in organic solvents, six resorcinarene molecules are known to form hexamers with an internal volume of around one cubic nanometer (nanocapsules) and shapes similar to the Archimedean solids.[2] Hydrogen bonds appear to hold the assembly together. A number of solvent or other molecules reside inside.[3] The resorcinarene is also the basic structural unit for other molecular recognition scaffolds, typically formed by bridging the phenolic oxygens with alkyl or aromatic spacers.[4] A number of molecular structures are based on this macrocycle, namely cavitands and carcerands.
Synthesis
The resorcinarene macrocycle is typically prepared by condensation of resorcinol and an aldehyde in concentrated acid solution.[5][6] Recrystallization typically gives the desired isomer in quite pure form. However, for certain aldehydes, the reaction conditions lead to significant by-products. Therefore, alternative condensation conditions have been developed, including the use of Lewis acid catalysts.
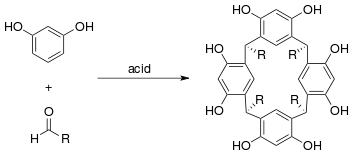
A green chemistry procedure was recently developed using solvent-free conditions: resorcinol, an aldehyde, and p-toluenesulfonic acid are ground together in a mortar and pestle at low temperature.[7] A paste-like solid forms upon sitting for several minutes, which can be washed with water and crystallized to give the desired resorcinarene.
Under each of these conditions, aromatic aldehydes react much faster, but less stereoselectively than aliphatic aldehydes, and the reaction frequently leads to a mixture of desired and undesired products.
Structure
Resorcinarenes can be characterized by a wide upper rim and a narrow lower rim. The upper rim includes eight hydroxyl groups that can participate in hydrogen bonding interactions. Depending on the aldehyde starting material, the lower rim includes four appending groups, usually chosen to give optimal solubility. The resorcin[n]arene nomenclature is analogous to that of calix[n]arenes, in which 'n' represents the number of repeating units in the ring. Pyrogallolarenes are related macrocycles derived from the condensation of pyrogallol (1,2,3-trihydroxybenzene) with an aldehyde.
History
During the late 19th century, Adolf von Baeyer noted that the condensation reaction of resorcinol and benzaldehyde gave a mixture of products that was beyond the characterization methods of that time. Over the following century, the product mixture was more fully characterized by X-ray crystallography and Nuclear Magnetic Resonance and found to include general structure shown above and several other stereoisomers.
Catalysis
The resorcinarene hexamer is extensively used as a yoctolitre reaction vessel for supramolecular catalysis due to its Bronsted acid nature.[8][9] Significant research in this area includes terpene cyclyzations, and iminium catalyzed reactions.[10][11]
References
- Aoyama Y, Tanaka Y, Toi H, Ogoshi H (1988). "Polar host–guest interaction. Binding of nonionic polar compounds with a resorcinol-aldehyde cyclooligomer as a lipophilic polar host". Journal of the American Chemical Society. 110 (2): 634–635. doi:10.1021/ja00210a073.
- Atwood JL, Barbour LJ, Jerga A (2002). "Organization of the interior of molecular capsules by hydrogen bonding". Proceedings of the National Academy of Sciences. 99 (8): 4837–4841. Bibcode:2002PNAS...99.4837A. doi:10.1073/pnas.082659799. PMC 122679. PMID 11943875.
- Shivanyuk A, Rebek J (2001). "Reversible encapsulation by self-assembling resorcinarene subunits". Proceedings of the National Academy of Sciences. 98 (14): 7662–7665. Bibcode:2001PNAS...98.7662S. doi:10.1073/pnas.141226898. PMC 35398. PMID 11427733.
- Jordan, J. H.; Gibb, B. C. (2017). "1.16 - Water-Soluble Cavitands☆". In Atwood, Jerry (ed.). Comprehensive Supramolecular Chemistry II. Elsevier. pp. 387–404. ISBN 9780128031995.
- Högberg AGS (1980). "Two stereoisomeric macrocyclic resorcinol-acetaldehyde condensation products". Journal of Organic Chemistry. 45 (22): 4498–4500. doi:10.1021/jo01310a046.
- Högberg AGS (1980). "Cyclooligomeric phenol-aldehyde condensation products. 2. Stereoselective synthesis and DNMR study of two 1,8,15,22-tetraphenyl[14]metacyclophan-3,5,10,12,17,19,24,26-octols". Journal of the American Chemical Society. 102 (19): 6046–6050. doi:10.1021/ja00539a012.
- Antesberger J, Cave GW, Ferrarelli MC, Heaven MW, Raston CL, Atwood JL (2005). "Solvent-free, direct synthesis of supramolecular nano-capsules". Chemical Communications. 2005 (7): 892–894. doi:10.1039/b412251h. PMID 15700072.CS1 maint: multiple names: authors list (link)
- Zhang, Qi; Tiefenbacher, Konrad (16 October 2013). "Hexameric Resorcinarene Capsule is a Brønsted Acid: Investigation and Application to Synthesis and Catalysis". Journal of the American Chemical Society. 135 (43): 16213–16219. doi:10.1021/ja4080375. PMID 24131349.
- Zhang, Qi; Catti, Lorenzo; Kaila, Ville R. I.; Tiefenbacher, Konrad (2017). "To catalyze or not to catalyze: elucidation of the subtle differences between the hexameric capsules of pyrogallolarene and resorcinarene". Chemical Science. 8 (2): 1653–1657. doi:10.1039/c6sc04565k. PMC 5364520. PMID 28451294.
- Zhang, Q.; Tiefenbacher, K. (16 February 2015). "Terpene cyclization catalysed inside a self-assembled cavity". Nature Chemistry. 7 (3): 197–202. Bibcode:2015NatCh...7..197Z. doi:10.1038/nchem.2181. PMID 25698327.
- Bräuer, Thomas M.; Zhang, Qi; Tiefenbacher, Konrad (27 June 2016). "Iminium Catalysis inside a Self-Assembled Supramolecular Capsule: Modulation of Enantiomeric Excess". Angewandte Chemie International Edition. 55 (27): 7698–7701. doi:10.1002/anie.201602382. PMID 27259076.
- Palmer LC, Shivanyuk A, Yamanaka M, Rebek J (2005). "Resorcinarene assemblies as synthetic receptors". Chemical Communications. 2005 (7): 857–858. doi:10.1039/b414252g. PMID 15700060.CS1 maint: multiple names: authors list (link)