Rhodotorula
Rhodotorula is a genus of unicellular pigmented yeasts, part of the division Basidiomycota. It is readily identifiable by distinctive orange/red colonies when grown on SDA (Sabouraud's Dextrose Agar). This distinctive color is the result of pigments that the yeast creates to block out certain wavelengths of light (620–750 nm) that would otherwise be damaging to the cell.
| Rhodotorula | |
|---|---|
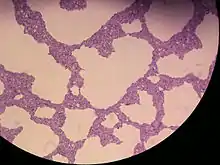 | |
| Rhodotorula mucilaginosa cells, Methylene blue stain, magnification 400x | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Rhodotorula F.C.Harrison (1927) |
| Type species | |
| Rhodotorula glutinis (Fresen.) F.C.Harrison (1928) | |
| Species | |
|
See text. | |
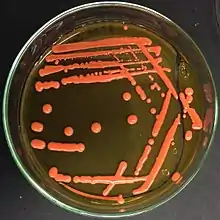
Habitat
Rhodotorula is a common environmental inhabitant. It can be cultured from soil, water, milk, fruit juice, and air samples.[1] It is able to scavenge nitrogenous compounds from its environment remarkably well, growing even in air that has been carefully cleaned of any fixed nitrogen contaminants. In such conditions, the nitrogen content of the dry weight of Rhodotorula can drop as low as 1%, compared to around 14% for most bacteria growing in normal conditions.[2]
Pathology
Only Rhodotorula mucilaginosa, R. glutinis, and R. minuta have been known to cause disease in humans. There were no reported cases of Rhodotorula infections before 1985.[1] There were however forty-three reported cases of Rhodotorula bloodstream infections (BSIs) between 1960 and 2000.[3] Rhodotorula is most commonly found in patients who are immunosuppressed and/or are using foreign-body technology such as central venous catheters.[3] Rhodotorula is commonly treated by removing the catheter and the use of anti-fungals. Rhodotorula is susceptible to amphotericin B and flucytosine.[3]
Rhodotorula can also cause infections in animals. There have been reports of skin infections in chickens and sea animals and lung infections and otitis in sheep and cattle.[1]
Potential in bioremediation
One area in which Rhodotorula species may become of importance is in bioremediation, especially of contaminated water sites. Although typically bacteria are commonly used bioremediation studies, there are more fungal studies now than ever. As with bacteria, fungi can naturally develop modified metabolism to deal with environmental contaminants, and could then be used in bioremediation. One main target is often polycyclic aromatic hydrocarbons (PAHs) since they often persist in the environment and have high levels of toxicity. Through sediment analysis and testing of contaminated waters Rhodotorula were found to be common in contaminated sites.[4] It was noted in samples taken from contaminated waters that Rhodotorula species had the ability to degrade petroleum compounds.[5] These studies as well as others suggest that Rhodotorula species may be good candidates for bioremediation of polluted waters for PAHs. In more directed studies a number of species of Rhodotorula were found to be able to degrade a number of specific contaminants. For example, R. glutinis and R. rubra have both been found to have a high ability to degrade phenanthrene,[6] while R. minuta has been found to degrade benzo(a)anthracene.[6] In a mixed fungal community Rhodotorula species contributed to effective degradation of low molecular weight PAHs, and although bacterial communities alone were not able to, the fungal communities also degraded high molecular weight PAHs (more than 3 benzene rings) such as chrysene and benzo(a)pyrene.[7] A strain of R. taiwanensis was shown to grow at constant gamma radiation 66 Gy/h at pH 2.3 and in the presence of high concentrations of mercury and chromium compounds, and forming biofilms under high-level chronic radiation and low pH, making it a promising candidate for bioremediation of acidic radioactive waste sites.[8]
Species
- Rhodotorula acheniorum
- Rhodotorula acuta
- Rhodotorula araucariae
- Rhodotorula armeniaca
- Rhodotorula aurantiaca
- Rhodotorula auriculariae
- Rhodotorula bacarum
- Rhodotorula benthica
- Rhodotorula biourgei
- Rhodotorula bogoriensis
- Rhodotorula bronchialis
- Rhodotorula buffonii
- Rhodotorula calyptogenae
- Rhodotorula cladiensis
- Rhodotorula cresolica
- Rhodotorula crocea
- Rhodotorula cycloclastica
- Rhodotorula dairenensis
- Rhodotorula evergladiensis
- Rhodotorula ferulica
- Rhodotorula foliorum
- Rhodotorula fragaria
- Rhodotorula fujisanensis
- Rhodotorula futronensis
- Rhodotorula gelatinosa
- Rhodotorula glacialis
- Rhodotorula glutinis
- Rhodotorula gracilis
- Rhodotorula graminis
- Rhodotorula grinbergsii
- Rhodotorula himalayensis
- Rhodotorula hinnulea
- Rhodotorula histolytica
- Rhodotorula hylophila
- Rhodotorula incarnata
- Rhodotorula ingeniosa
- Rhodotorula javanica
- Rhodotorula koishikawensis
- Rhodotorula lactosa
- Rhodotorula lamellibrachiae
- Rhodotorula laryngis
- Rhodotorula lignophila
- Rhodotorula lini
- Rhodotorula longissima
- Rhodotorula ludwigii
- Rhodotorula lysinophila
- Rhodotorula marina
- Rhodotorula matritensis
- Rhodotorula meli
- Rhodotorula minuta
- Rhodotorula mucilaginosa
- Rhodotorula nitens
- Rhodotorula nothofagi
- Rhodotorula oryzae
- Rhodotorula pacifica
- Rhodotorula pallida
- Rhodotorula philyla
- Rhodotorula pilimanae
- Rhodotorula pinicola
- Rhodotorula psychrophenolica
- Rhodotorula psychrophila
- Rhodotorula pustula
- Rhodotorula retinophila
- Rhodotorula rosulata
- Rhodotorula rubra
- Rhodotorula rubrorugosa
- Rhodotorula silvestris
- Rhodotorula sinensis
- Rhodotorula slooffiae
- Rhodotorula sonckii
- Rhodotorula straminea
- Rhodotorula subericola
- Rhodotorula terpenoidalis
- Rhodotorula terrea
- Rhodotorula tokyoensis
- Rhodotorula ulzamae
- Rhodotorula vanillica
- Rhodotorula vuilleminii
- Rhodotorula yarrowii
- Rhodotorula yunnanensis
- Rhodotorula zsoltii
References
- Wirth F, Goldani LZ (September 2012). "Epidemiology of Rhodotorula: an emerging pathogen". Interdisciplinary Perspectives on Infectious Diseases. 2012: 465717. doi:10.1155/2012/465717. PMC 3469092. PMID 23091485.
- Postgate, John: "The Outer Reaches of Life", page 132-134. Cambridge University Press, 1994
- Zaas AK, Boyce M, Schell W, Lodge BA, Miller JL, Perfect JR (November 2003). "Risk of fungemia due to Rhodotorula and antifungal susceptibility testing of Rhodotorula isolates". Journal of Clinical Microbiology. 41 (11): 5233–5. doi:10.1128/jcm.41.11.5233-5235.2003. PMC 262498. PMID 14605170.
- Zhang H, Huang T, Chen S (February 2015). "Ignored sediment fungal populations in water supply reservoirs are revealed by quantitative PCR and 454 pyrosequencing". BMC Microbiology. 15: 44. doi:10.1186/s12866-015-0379-7. PMC 4349462. PMID 25886005.
- Bogusławska-Was E, Dabrowski W (July 2001). "The seasonal variability of yeasts and yeast-like organisms in water and bottom sediment of the Szczecin Lagoon". International Journal of Hygiene and Environmental Health. 203 (5–6): 451–8. doi:10.1078/1438-4639-00056. PMID 11556149.
- MacGillivray AR, Shiaris MP (May 1993). "Biotransformation of polycyclic aromatic hydrocarbons by yeasts isolated from coastal sediments". Applied and Environmental Microbiology. 59 (5): 1613–8. doi:10.1128/AEM.59.5.1613-1618.1993. PMC 182127. PMID 8517753.
- Hesham A, Khan S, Tao Y, Li D, Zhang Y, Yang M (September 2012). "Biodegradation of high molecular weight PAHs using isolated yeast mixtures: application of meta-genomic methods for community structure analyses". Environmental Science and Pollution Research International. 19 (8): 3568–78. doi:10.1007/s11356-012-0919-8. PMID 22535224. S2CID 8263841.
- Tkavc R, Matrosova VY, Grichenko OE, Gostinčar C, Volpe RP, Klimenkova P, Gaidamakova EK, Zhou CE, Stewart BJ, Lyman MG, Malfatti SA, Rubinfeld B, Courtot M, Singh J, Dalgard CL, Hamilton T, Frey KG, Gunde-Cimerman N, Dugan L, Daly MJ (2017). "Rhodotorula taiwanensis MD1149". Frontiers in Microbiology. 8: 2528. doi:10.3389/fmicb.2017.02528. PMC 5766836. PMID 29375494.
