Samer Hattar
Samer Hattar (Arabic سامر حتر ) is a chronobiologist and a leader in the field of non-image forming photoreception. He is currently the Chief of the Section on Light and Circadian Rhythms at the National Institute of Mental Health, part of the National Institutes of Health. He was previously an associate professor in the Department of Neuroscience and the Department of Biology at Johns Hopkins University in Baltimore, MD. He is best known for his investigation into the role of melanopsin and intrinsically photosensitive retinal ganglion cells (ipRGC) in the entrainment of circadian rhythms.
Samer Hattar (Arabic سامر حتر ) | |
|---|---|
| Born | |
| Alma mater | Yarmouk University |
| Known for | Melanopsin ipRGC |
| Awards | Alfred P. Sloan Research Fellow Lucile & David Packard Foundation Fellowship for Science & Engineering Albert Lehninger Research Award |
| Scientific career | |
| Fields | Neuroscience Chronobiology |
| Institutions | Johns Hopkins University American University of Beirut University of Houston |
Life
Samer Hattar was born in Amman, Jordan to a Jordanian father and a Lebanese mother. Raised in a Christian family, he planned on becoming a priest. He studied at Terra Sancta High School, a Catholic high school in Amman, from 1978-1988. He earned good grades in his classes and fell in love with biology when introduced to Mendel's pea plant experiments. This passion inspired him to pursue a career in science. He attended Yarmouk University in Irbid for his undergraduate studies, where he majored in Biology and minored in Chemistry. His high marks earned him the honor of meeting Hassan Bin Talal, the prince of Jordan. After graduating from Yarmouk in 1991, he completed a master's degree in biochemistry at the American University of Beirut in Beirut. He began his graduate studies in biochemistry in 1993 at the University of Houston where he studied circadian regulation of a transcription factor in aplysia.[1] Hattar completed his postdoctoral fellowship at the Solomon Snyder Department of Neuroscience at Johns Hopkins University School of Medicine, where he made discoveries on ipRGCs. In 2004, he established his laboratory in the Department of Biology at Johns Hopkins University.[2][3] He is married to Rejji Kuruvilla, a neuroscientist also working at Johns Hopkins.[4]
Scientific work
Hattar is known for his work in the area of chronobiology. He is credited with discovering that the photopigment melanopsin and associated ipRGCs play an important role in the entrainment of circadian rhythms [5][6][7] Before Hattar's work, it was assumed that organisms entrained to daily light-dark cycles through the same mechanisms that are responsible for vision. However, case studies reported that some who were completely blind could still entrain to these cycles. This observation, coupled with the discovery of melanopsin by Ignacio Provencio, led Hattar to hypothesize that this photopigment might be responsible for photoentrainment.[3]
Melanopsin as a circadian photopigment
In 2002, Hattar and his colleagues showed that melanopsin plays a key role in a variety of photic responses, including pupillary light reflex, and synchronization of the biological clock to daily light-dark cycles. He also described the role of melanopsin in ipRGCs. Using a rat melanopsin gene, a melanopsin-specific antibody, and fluorescent immunocytochemistry, the team concluded that melanopsin is expressed in some RGCs. Using a Beta-galactosidase assay, they found that these RGC axons exit the eyes together with the optic nerve and project to the suprachiasmatic nucleus (SCN), the primary circadian pacemaker in mammals. They also demonstrated that the RGCs containing melanopsin were intrinsically photosensitive. Hattar concluded that melanopsin is the photopigment in a small subset of RGCs that contributes to the intrinsic photosensitivity of these cells and is involved in their non-image forming functions, such as photic entrainment and pupillary light reflex.[8]
Melanopsin cells relay inputs from rods and cones
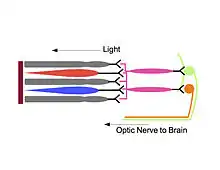
Hattar, armed with the knowledge that melanopsin was the photopigment responsible for the photosensitivity of ipRGCs, set out to study the exact role of the ipRGC in photoentrainment. In 2008, Hattar and his research team transplanted diphtheria toxin genes into the mouse melanopsin gene locus to create mutant mice that lacked ipRGCs. The research team found that while the mutants had little difficulty identifying visual targets, they could not entrain to light-dark cycles. These results led Hattar and his team to conclude that ipRGCs do not affect image-forming vision, but significantly affect non-image forming functions such as photoentrainment.[8]
Distinct ipRGCs
Further research has shown that ipRGCs project to different brain nuclei to control both non-image forming and image forming functions.[9] These brain regions include the SCN, where input from ipRGCs is necessary to photoentrain circadian rhythms, and the olivary pretectal nucleus (OPN), where input from ipRGCs control the pupillary light reflex.[10] Hattar and colleagues conducted research that demonstrated that ipRGCs project to hypothalamic, thalamic, stratal, brainstem and limbic structures.[11] Although ipRGCs were initially viewed as a uniform population, further research revealed that there are several subtypes with distinct morphology and physiology.[9] Since 2011, Hattar's laboratory[12] has contributed to these findings and has successfully distinguished subtypes of ipRGCs.[10]
Diversity of ipRGCs
Hattar and colleges utilized Cre-based strategies for labeling ipRGCs to reveal that there are at least five ipRGC subtypes that project to a number of central targets.[10] Five classes of ipRGCs, M1 through M5, have been characterized to date in rodents. These classes differ in morphology, dendritic localization, melanopsin content, electrophysiological profiles, and projections.[9]
Diversity in M1 cells
Hattar and his co-workers discovered that, even among the subtypes of ipRGC, there can be designated sets that differentially control circadian versus pupillary behavior. In experiments with M1 ipRGCs, they discovered that the transcription factor Brn3b is expressed by M1 ipRGCs that target the OPN, but not by ones that target the SCN. Using this knowledge, they designed an experiment to cross Melanopsin-Cre mice with mice that conditionally expressed a toxin from the Brn3b locus. This allowed them to selectively ablate only the OPN projecting M1 ipRGCS, resulting in a loss of pupil reflexes. However, this did not impair circadian photo entrainment. This demonstrated that the M1 ipRGC consist of molecularly distinct subpopulations that innervate different brain regions and execute specific light-induced functions.[10] This isolation of a 'labeled line' consisting of differing molecular and functional properties in a highly specific ipRGC subtype was an important first for the field. It also underscored the extent to which molecular signatures can be used to distinguish between RGC populations that would otherwise appear the same, which in turn facilitates further investigation into their specific contributions to visual processing.[10]
Psychological impact of light exposure
Previous studies in circadian biology have established that exposure to light during abnormal hours leads to sleep deprivation and disruption of the circadian system, which affect mood and cognitive functioning. While this indirect relationship had been corroborated, not much work had been done to examine whether there was a direct relationship between irregular light exposure, aberrant mood, cognitive function, normal sleep patterns and circadian oscillations. In a study published in 2012, the Hattar Laboratory was able to show that deviant light cycles directly induce depression-like symptoms and lead to impaired learning in mice, independent of sleep and circadian oscillations.[13]
Effect on mood
ipRGCs project to areas of the brain that are important for regulating circadian rhythmicity and sleep, most notably the SCN, subparaventricular nucleus, and the ventrolateral preoptic area. In addition, ipRGCs transmit information to many areas in the limbic system, which is strongly tied to emotion and memory. To examine the relationship between deviant light exposure and behavior, Hattar and his colleagues studied mice exposed to alternating 3.5-hour light and dark periods (T7 mice) and compared them with mice exposed to alternating 12-hour light and dark periods (T24 mice). Compared to a T24 cycle, the T7 mice got the same amount of total sleep and their circadian expression of PER2, an element of the SCN pacemaker, was not disrupted. Through the T7 cycle, the mice were exposed to light at all circadian phases. Light pulses presented at night lead to expression of the transcription factor c-Fos in the amygdala, lateral habenula, and subparaventricular nucleus further implicating light’s possible influence on mood and other cognitive functions.[14]
Mice subjected to the T7 cycle exhibited depression-like symptoms, exhibiting decreased preference for sucrose (sucrose anhedonia) and exhibiting more immobility than their T24 counterparts in the forced swim test (FST). Additionally, T7 mice maintained rhythmicity in serum corticosterone, however the levels were elevated compared to the T24 mice, a trend that is associated with depression. Chronic administration of the antidepressant Fluoxetine lowered corticosterone levels in T7 mice and reduced depression-like behavior while leaving their circadian rhythms unaffected.[13]
Effect on learning
The hippocampus is a structure in the limbic system that receives projections from ipRGCs. It is required for the consolidation of short-term memories into long-term memories as well as spatial orientation and navigation. Depression and heightened serum corticosterone levels are linked to impaired hippocampal learning. Hattar and his team analyzed the T7 mice in the Morris water maze (MWM), a spatial learning task that places a mouse in a small pool of water and tests the mouse’s ability to locate and remember the location of a rescue platform located just below the waterline. Compared to the T24 mice, the T7 mice took longer to find the platform in subsequent trials and did not exhibit a preference for the quadrant containing the platform. In addition, T7 mice exhibited impaired hippocampal long-term potentiation (LTP) when subjected to theta burst stimulation (TBS). Recognition memory was also affected, with T7 mice failing to show preference for novel objects in the novel object recognition test.[15]
Necessity of ipRGCs
Mice without (Opn4aDTA/aDTA mice) are not susceptible to the negative effects of an aberrant light cycle, indicating that light information transmitted through these cells plays an important role in regulation of mood and cognitive functions such as learning and memory.[16]
Awards and honors
References
- "NACS Event :: Atypical mammalian photoreceptors influence circadian rhythms, mood and learning". NACS: Program in Neuroscience and Cognitive Science, University of Maryland, College Park. Archived from the original on 4 September 2014. Retrieved 12 April 2013.
- "Samer Hattar - Biology". Johns Hopkins University. Archived from the original on 2014-09-04.
- Hendricks, Melissa. "Clock Wise". The Johns Hopkins Magazine. Johns Hopkins University. Retrieved 22 April 2015.
- "Rejji Kuruvilla". Department of Biology. Johns Hopkins University. Retrieved 27 December 2016.
- Reppert, Stephen; Weaver, D. R. (29 August 2002). "Coordination of circadian timing in mammals". Nature. 418 (6901): 935–941. Bibcode:2002Natur.418..935R. doi:10.1038/nature00965. PMID 12198538.
- Schmidt, Tiffany; Do, Michael; Dacey, Dennis; Lucas, Robert; Hattar, Samer; Matynia, Anna (9 November 2011). "Melanopsin-Positive Intrinsically Photosensitive Retinal Ganglion Cells: From Form to Function". The Journal of Neuroscience. 31 (45): 16094–16101. doi:10.1523/JNEUROSCI.4132-11.2011. PMC 3267581. PMID 22072661.
- Sansoni, Paola; Mercatelli, Luca; Farini, Alessandro (2015). Sustainable Indoor Lighting. Springer. p. 290. ISBN 9781447166337. Retrieved 8 April 2015.
- Graham, Dustin. "Melanopsin Ganglion Cells: A Bit of Fly in the Mammalian Eye". Webvision The Organization of the Retina and Visual System. University of Utah School of Medicine. Archived from the original on 27 April 2011. Retrieved 9 April 2015.
- Matynia, Anna (September 3, 2013). "Blurring the boundaries of vision: novel functions of intrinsically photosensitive retinal ganglion cells". Journal of Experimental Neuroscience. 7: 43–50. doi:10.4137/JEN.S11267. PMC 4089729. PMID 25157207.
- Dhande, OS; Huberman, AD (November 19, 2013). "Retinal Ganglion Cell Maps in the Brain: Implications for Visual Processing". Current Opinion in Neurobiology. 24 (1): 133–142. doi:10.1016/j.conb.2013.08.006. PMC 4086677. PMID 24492089.
- Gaggioni G; Maquet P; Schmidt C; Dijk Dj; Vandealle G (July 8, 2014). "Neuroimaging, Cognition, Light and Circadian Rhythms". Frontiers in Systems Neuroscience. 8: 126. doi:10.3389/fnsys.2014.00126. PMC 4086398. PMID 25071478.
- "The Hattar Lab". Johns Hopkins University. 2014. Retrieved 27 December 2016.
- Dulcis, Davide; Jamshidi, Pouya; Leutgeb, Stefan; Spitzer, Nicholas C. (26 April 2013). "Neurotransmitter Switching in the Adult Brain Regulates Behavior". Science. 340 (6131): 449–453. Bibcode:2013Sci...340..449D. doi:10.1126/science.1234152. PMID 23620046.
- Masana, MI (December 1996). "Light-induced c-fos mRNA expression in the suprachiasmatic nucleus and the retina of C3H/HeN mice". Molecular Brain Research. 42 (2): 193–201. doi:10.1016/s0169-328x(96)00031-9. PMID 9013774.
- Sauer, Jonas-Frederic (3 March 2015). "Impaired fast-spiking interneuron function in a genetic mouse model of depression". eLife. 4. doi:10.7554/elife.04979. PMC 4374525. PMID 25735038.
- Monteggia, Lisa; Kavalali, E. T. (2012). "Circadian rhythms: Depression brought to light". Nature. 491 (7425): 537–538. doi:10.1038/nature11752. PMID 23151474.
- "The Young Investigators, April 8, 2004". Dome | Johns Hopkins Medicine. Archived from the original on 2004-07-07.
- http://www.bio.jhu.edu/Directory/FacultyHonors.aspx