Saturated and unsaturated compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word saturare, meaning 'to fill')[1]
Organic chemistry
Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols.
| Saturated compounds | |
| Ethane | Propane |
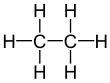 |
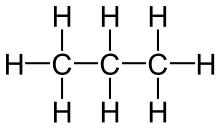 |
| 1-Octanol | |
| Saturated fatty acids | |
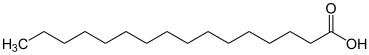 | |
Unsaturated organic compounds
The concept of saturation can be described using various naming systems, formulas, and analytical tests. For instance, IUPAC nomenclature is a system of naming conventions used to describe the type and location of unsaturation within organic compounds. The "degree of unsaturation" is a formula used to summarize and diagram the amount of hydrogen that a compound can bind. Unsaturation can be determined by NMR, mass spectrometry and IR spectroscopy, or by determining a compound's bromine number or iodine number.[2]
| Unsaturated compounds | |
|---|---|
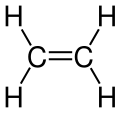
|
|
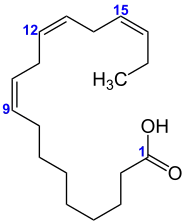
| |
Fatty acids
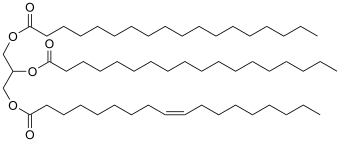
The terms saturated vs unsaturated are often applied to the fatty acid constituents of fats. The triglycerides (fats) that comprise tallow are derived from the saturated stearic and monounsaturated oleic acids.[3] Many vegetable oils contain fatty acids with one (monounsaturated) or more (polyunsaturated) double bonds in them.
Saturated and unsaturated compounds beyond organic chemistry
Organometallic chemistry
In organometallic chemistry, a coordinatively unsaturated complex has fewer than 18 valence electrons and thus is susceptible to oxidative addition or coordination of an additional ligand. Unsaturation is characteristic of many catalysts. The opposite of coordinatively unsaturated is coordinatively saturated. Complexes that are coordinatively saturated rarely exhibit catalytic properties.[4][5]
Surfaces
In physical chemistry, when referring to surface processes, saturation denotes the degree at which a binding site is fully occupied. For example, base saturation refers to the fraction of exchangeable cations that are base cations.
References
- Mosby's Medical, Nursing & Allied Health Dictionary, Fourth Edition, Mosby-Year Book Inc., 1994, p. 1394
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Alfred Thomas (2002). "Fats and Fatty Oils". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_173.
- Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 1-891389-53-X
- "IUPAC definition of Coordinatively Unsaturated Complex".