Scenedesmus
Scenedesmus is a genus of green algae, in the class Chlorophyceae. They are colonial and non-motile.
| Scenedesmus | |
|---|---|
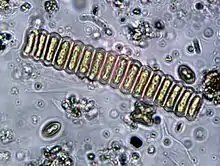 | |
| Scenedesmus bijunga | |
| Scientific classification | |
| Phylum: | Chlorophyta |
| Class: | Chlorophyceae |
| Order: | Sphaeropleales |
| Family: | Scenedesmaceae |
| Genus: | Scenedesmus Meyen, 1829 |
| Type species | |
| Scenedesmus obtusus Meyen, 1829 | |
| Species | |
| |
Taxonomy
Currently, there are 74 taxonomically accepted species of Scenedesmus.[1] Additionally, several subgenera have been identified, but vary according to the source. Hegewald denotes Acutodesmus, Desmodesmus, and Scenedesmus as the three major categories. Acutodesmus is characterized as having acute cell poles, while Desmodesmus and Scenedesmus have obtuse/truncated cell poles (differentiated by the presence or absence of spines respectively). Fossil records date Scenedesmus from 70 to 100 million years ago with Desmodesmus suspected to be the youngest of these three groups.[2]
Basic biology
Scenedesmus is one of the most common freshwater algae genera; however, the extremely diverse morphologies found within species make identification difficult.[3] While most species are found across the world, certain species exist only in local populations such as S. intermedius and S. serratus which are found in New Zealand.[3]
Coenobia and cell growth
Scenedesmus can exist as unicells; they are also frequently found in coenobia of four or eight cells[3] inside a parental mother wall. Various coenobial architectures have been described, including linear, costulatoid, irregular, alternating, or dactylococcoid patterns (Figure 1).[3] The formation of coenobia is dependent on a number of factors. A higher proportion of unicellular organisms was found at high light intensities and high temperatures, suggesting that at higher growth rates the organisms prefer to be non-colonized.[3] Successful growth and division for algae relies on a balance between maintaining buoyancy in the euphotic zone (containing ideal light and nutritional conditions) and avoidance of grazing predators.[3] Larger colonies have a smaller surface-to-volume ratio, which limits nutrient uptake and light harvesting, and the large mass promotes sinking. However, in the presence of grazers, such as Daphnia, that threaten to consume unicellular algae, the larger colonies provide significant security.[3] This threat can be so significant that the cells will coalesce into these 8-cell colonies even in severely limiting growth conditions in order to reduce grazing vulnerability or while in nutrient-deplete conditions.[3][4]
Defense mechanisms
The cells have other mechanisms of self-defense in addition to colonizing. Scenedesmus can be divided into two subgenera, the non-spiny Scenedesmus and the spiny Desmodesmus. Although spineless, the Scenedesmus subgenera cells have thick cells walls and mucilage, which may make them digestion-resistant. Some chemical compounds in Scenedesmus could even be toxic to certain organisms upon consumption. Bristles of up to 100 um may form a net in both spiny and non-spiny varieties to discourage predation even further.[3] Cells defensively form these bristles when kairomones are detected, an infochemical released by Daphnia that Scenedesmus has evolved to recognize as a warning signal.[3][4]
Reproduction and colony formation
During replication, the mother cell enlarges and becomes multinucleate after multiple divisions. The cytoplasm then is cleaved into uninucleate daughter cells, usually developing as non-motile autospores. These daughter cells typically link up with other daughter cells to form a colony within the parental cell wall to be later released.[5] The cells progress through a typical mitotic cycle similar to other members of Chlorophyceae, with the cytoplasm of the daughter cells becoming very dense.[5] Eventually the mother cell wall breaks and releases the spores which adopt a normal cellular appearance.[5] The cells at either end of the coenobium are different in morphology from those in the center.[5] How the cells adhere to one another during development is still unclear, but it is known that a trilaminar sheath (TLS), composed of algaenan, is one of the first exterior structures to form, developing in patches before growing to connect into one continuous layer.[5] The ornamented layer is the last component to develop.[5]
Cell ornamentation and outer layers
The exterior ornamentation is highly variable within the genus Scenedesmus. Staehelin et al. characterized two species in detail: S. pannonicus and S. longus. S. pannonicus assembles a tight-fitting "warty" layer compared to the loose "reticulate" layer found on S. longus.[6] A shared feature between the two is a TLS found at the junction between neighboring cells that helps cement them together.[6] An additional pectic layer observed on S. pannonicus forms a thick mesh of thin filaments originating from the warty layer.[6] Another feature of the outer coenobial surface of S. pannonicus is a combination of individual spikes (seemingly connected to the warts) and small spikelets that fuse to form combs that zigzag along the cell.[6] An overview of these structures can be seen in Figure 2. The last major category of ornamentation is rosettes that are common to many Scenedesmus species.[6] Rosettes are ring-shaped structures enclosing small mounds on the cell surface and are usually sitting upon a thicker layer of cell wall than the surrounding areas.[6] No potential function for these structures has been suggested. While S. longus was not observed with the comb-like structures of S. pannonicus, it did have two variations of spikelets forming between the TLS and reticulate layer to keep the two apart.[6]
Mitochondrial DNA
Scenedesmus obliquus is notable for the non-standard coding of its mitochondrial DNA which may represent an intermediate form in the evolution of green algal mitochondrial DNA.[7] This code is represented by NCBI translation table 22, Scenedesmus obliquus mitochondrial code.[8]
Biofuel production
Although Scenedesmus is capable of producing many kinds of biofuels such as bio-hydrogen, biodiesel, bioethanol and drop-in fuels, most extensive research has been done on the use of Scenedesmus for bio-diesel production. Like all algae systems, the implementation of integrated biofuel production of Scenedesmus from the laboratory findings has challenges in large-scale production. Major challenges include nutrient supply and recycling, gas transfer and exchange, PAR (Photosynthetically Active Radiation) delivery, cultural integrity, environmental control, land and water availability, harvesting, and genetic and metabolic engineering[9]
Bio-hydrogen (H2) production
In 1942, Gaffron and Rubin may be credited with conducting an experiment that sparked H2 production research in green algae using Scenedesmus obliquus.[10] Algae produce H2 gas under anaerobic conditions by providing hydrogenases with hydrogen ions derived from splitting of water molecules via photosynthesis.[11] However, enzyme activity is transient due to inhibition from O2 production via photosynthesis, a problem that continues to plague H2 production.[12] S. obliquus is traditionally known to utilize a nickel-iron hydrogenase, but usage of other iron hydrogenases in H2 production is also reported.[13] Hydrogenase enzyme activity in Scenedesmus species is reported to be lower than that of Chlamydomonas reinhardtii.[14] H2 production independent of Photosystem II in Scenedesmus has also been performed using redox equivalents of fermentative metabolism under dark anaerobic incubation.[10][15] Research findings suggest that a sulfur-deprived environment triggers an imbalance in the photosynthesis and respiration relationship, resulting in net consumption of O2, causing anaerobiosis, and switching to hydrogen production.[12] Ultrasonication pretreatment has been effective in increasing fermentative bioenergy production from Scenedesmus oliquus YSW15.[16] Bio-hydrogen production research using Scenedesmus is actively spurred by its applications to wastewater treatment. (See subsequent section on waste management by Scenedesmus).
Bio-diesel production
Scenedesmus is known to have high biomass productivity among green algae, and has been actively researched for its use for bio-diesel production. Its heterotrophic production of biomass and lipid in optimized conditions is reported to have higher efficiency than its autotrophic production.[17][18][19][20] Optimization of biomass productivity as well as lipid content through varying concentration of supplemental nutrients has been done in numerous studies; currently, Scenedesmus lipid yield after optimization has reached ~60% dry cell weight, lower than some other algae.[18][21] However, Scenedesmus is more efficient at capturing CO2 than other algae.[20] Like many algae species, Scenedesmus required nitrate-deficient condition to profoundly increase its lipid yield.[22] A significant improvement (up to six-fold) of feedstock yields was achieved by adding varying concentrations of ethanol under a 12-hr photoperiod and in the dark.[22] The most significant improvement in lipid production was obtained when stationary phase cultures were transferred to media deficient in nitrate for 7 days and phosphate for 3 days, respectively.[18] Extraction of oils with methanol or ethanol from the Scenedesmus remains a challenge and its lower lipid content adds to the cost of production.[18] In a recent study,[23] Scenedesmus abundans was isolated from Dal Lake, Kashmir and proved to be a suitable raw material for biodiesel production. The alga increased significantly in biomass and lipid content with the nitrogen concentration of 0.32g/L of nitrogen. A two-step transesterification was found to be best suited for transesterification, while Folch extraction was best for lipid extraction.[23]
Bio-ethanol
Scenedesmus, and other microalgae such as Chlorella, Dunaliella, Chlamydomonas, and Spirulina, contain large amounts of carbohydrate (>50% of the dry weight), which make them attractive candidates for bio-ethanol production.[24] In one study,[25] Scenedesmus was used to yield high biomass productivity; its carbohydrate-rich biomass was then hydrolyzed with 2% sulfuric acid and underwent an SHF (Separate Hydrolysis and Fermentation) process to produce 8.55 g L−1 of ethanol and a maximum yield of 0.213 g ethanol / g biomass within 4 hours of ethanol fermentation.
Drop-in fuels
Isoprenoids are considered important metabolites that can be utilized as drop-in fuels, often as alkane chains. Scenedesmus conducts a pyruvate/glyeraldehyde 3-phosphate non-mevalonate pathway to synthesize isoprenoids. However, isoprenoid yields were too low (1.5~15 mg per 10 liter of Scenedesmus culture when cells reached 0.5-0.6 g L−1) to be considered viable for future drop-in fuel production.[26]
Wastewater management
In a study comparing the efficiency of ammonia and phosphorus removal from an agroindustrial wastewater by Chlorella vulgaris and Scenedesmus dimorphus, Scenedesmus exhibited better efficiency of removing ammonia in cylindrical bioreactor while both algae removed phosphorus from the wastewater to the same extent.[27] Algal Turf Scrubber (ATS) is one of many technologies that utilize algae for treating variety of wastes and industrially polluted waters.[28] An algal turf scrubber in Florida, for example, removed phosphorus at a cost of $24 per kg whereas engineered wetland processes removed phosphorus at a cost of $77 per kg. While removing metallic wastes as well as organic substrates, growing Scenedesmus biomass could be utilized for producing cattle feeds, organic fertilizers, paper, construction paper, and biodiesel.[9]
Image gallery
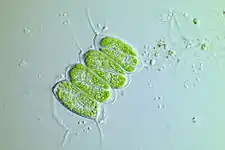
 Scenedesmus quadricauda
Scenedesmus quadricauda Scenedesmus brasiliensis
Scenedesmus brasiliensis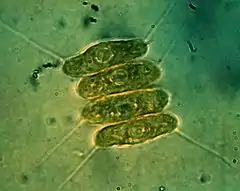

References
- M.D. Guiry in Guiry, M.D. & Guiry, G.M. 2015. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 16 April 2015.
- Hegewald, Eberhard H. "Taxonomy and Phylogeny of Scenedesmus." The Korean Journal of Phycology 12.4 (1997): 235-46.
- Lürling, Miquel. The Smell of Water: Grazer-Induced Colony Formation in Scenedesmus. Thesis. Agricultural University of Wageningen, 1999.
- Lürling Miquel; Van Donk Ellen (2000). "Grazer-Induced Colony Formation in Scenedesmus: Are There Costs to Being Colonial?". Oikos. 88 (1): 111–118. doi:10.1034/j.1600-0706.2000.880113.x.
- Pickett-Heaps Jeremy D.; Staehelin L. Andrew (1975). "The Ultrastructure of Scenedesmus (Chlorophyceae). II. Cell Division and Colony Formation". Journal of Phycology. 11 (2): 186–202. doi:10.1111/j.1529-8817.1975.tb02766.x.
- Staehelin L. Andrew; Pickett-Heaps Jeremy D. (1975). "The Ultrastructure of Scenedesmus (Chlorophyceae). I. Species With The "Reticulate" or "Warty" Type of Ornamental Layer". Journal of Phycology. 11 (2): 163–85. doi:10.1111/j.1529-8817.1975.tb02765.x.
- A. M. Nedelcu; R. W. Lee; G. Lemieux; M. W. Gray; G. Burger (June 2000). "The complete mitochondrial DNA sequence of Scenedesmus obliquus reflects an intermediate stage in the evolution of the green algal mitochondrial genome". Genome Research. 10 (6): 819–31. doi:10.1101/gr.10.6.819. PMC 310893. PMID 10854413.
- Elzanowski A, Ostell J, Leipe D, Soussov V. "The Genetic Codes". Taxonomy browser. National Center for Biotechnology Information (NCBI), U.S. National Library of Medicine. Retrieved 11 August 2016.
- Christenson Logan, Sims Ronald (2011). "Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts". Biotechnology Advances. 29 (6): 686–702. doi:10.1016/j.biotechadv.2011.05.015. PMID 21664266.
- Timmins Matthew; et al. (2009). "Phylogenetic and molecular analysis of hydrogen-producing green algae". Journal of Experimental Botany. 60 (6): 1691–1702. doi:10.1093/jxb/erp052. PMC 2671627. PMID 19342428.
- Kapdan Ilgi Karapinar, Kargi Fikret (2006). "Bio-hydrogen production from waste materials". Enzyme and Microbial Technology. 38 (5): 569–582. doi:10.1016/j.enzmictec.2005.09.015.
- Melis, Anastasios, and Thomas Happe. "Hydrogen production. Green algae as a source of energy." Plant physiology 127.3 (2001): 740-748.
- Florin, Lore, Anestis Tsokoglou, and Thomas Happe. "A Novel Type of Iron Hydrogenase in the Green Alga Scenedesmus obliquus is Linked to the Photosynthetic Electron Transport Chain." Journal of Biological Chemistry276.9 (2001): 6125-6132.
- Winkler M, Hemschemeier A, Gotor C, Melis A, Happer T. [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int J Hydrogen Energy 2002;27:1431-9.
- Das Debabrata; Veziroǧlu T. Nejat (2001). "Hydrogen production by biological processes: a survey of literature". International Journal of Hydrogen Energy. 26 (1): 13–28. doi:10.1016/s0360-3199(00)00058-6.
- Choi, Jeong-A., et al. "Enhancement of fermentative bioenergy (ethanol/hydrogen) production using ultrasonication of Scenedesmus obliquus YSW15 cultivated in swine wastewater effluent." Energy & Environmental Science (2011); 4(9): 3513-3520.
- El-Sheekh, Mostafa, Abd El-Fatah Abomohra, and Dieter Hanelt. "Optimization of biomass and fatty acid productivity of Scenedesmus obliquus as a promising microalga for biodiesel production." World Journal of Microbiology and Biotechnology (2012): 1-8.
- Mandal Shovon, Mallick Nirupama (2009). "Microalga Scenedesmus obliquus as a potential source for biodiesel production". Applied Microbiology and Biotechnology. 84 (2): 281–291. doi:10.1007/s00253-009-1935-6. PMID 19330327.
- da Silva, Teresa Lopes, et al. "Oil production towards biofuel from autotrophic microalgae semicontinuous cultivations monitorized by flow cytometry." Applied biochemistry and biotechnology 159.2 (2009): 568-578.
- Yoo Chan; et al. (2010). "Selection of microalgae for lipid production under high levels carbon dioxide". Bioresource Technology. 101 (1): S71–S74. doi:10.1016/j.biortech.2009.03.030. PMID 19362826.
- Banerjee, Anirban, et al. "Botryococcus braunii: a renewable source of hydrocarbons and other chemicals." Critical reviews in biotechnology 22.3 (2002): 245-279.
- Wu, Chengchen, et al. "Enhancement Effect of Ethanol on Lipid and Fatty Acid Accumulation and Composition of Scenedesmus sp." Bioresource Technology (2013).
- Mandotra S.K., Kumar Pankaj, Suseela M.R., Ramteke P.W. (2014). "Fresh water green microalga Scenedesmus abundans: A potential feedstock for high quality biodiesel production". Bioresource Technology. 156: 42–47. doi:10.1016/j.biortech.2013.12.127. PMID 24486936.CS1 maint: multiple names: authors list (link)
- John Rojan P; et al. (2011). "Micro and macroalgal biomass: a renewable source for bioethanol". Bioresource Technology. 102 (1): 186–193. doi:10.1016/j.biortech.2010.06.139. PMID 20663661.
- Ho, Shih-Hsin, et al. "Bioprocess development on microalgae-based CO 2 fixation and bioethanol production using Scenedesmus obliquus CNW-N." Bioresource Technology (2013).
- Schwender, J., et al. "Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus." Biochemical Journal 316.Pt 1 (1996): 73.
- González , Estela Luz, Olivia Cañizares Rosa, Baena Sandra (1997). "Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus". Bioresource Technology. 60 (3): 259–262. CiteSeerX 10.1.1.316.6465. doi:10.1016/s0960-8524(97)00029-1.CS1 maint: multiple names: authors list (link)
- http://www.algalturfscrubber.com/