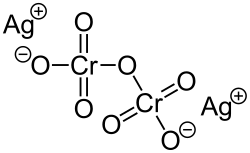
Silver dichromate
Silver dichromate is a chemical compound with the formula Ag2Cr2O7. It is insoluble in water and decomposes when treated with hot water. Its anion has a charge of -2.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Silver dichromate | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.131 | ||
| EC Number |
| ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| Ag2Cr2O7 | |||
| Molar mass | 431.76 g/mol | ||
| Appearance | ruby red powder | ||
| Density | 4.77 g/cm3 | ||
| Ksp = 2.0×10−7 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
- K2Cr2O7 (aq) + 2 AgNO3 (aq) + Ag2Cr2O7 (s) + 2 KNO3 (aq)
Applications
Related complexes are used as oxidants in organic chemistry.[1] For instance, tetrakis(pyridine)silver dichromate, [Ag2(py)4]2+[Cr2O7]2−, is used to convert benzylic and allylic alcohols to corresponding carbonyl compounds.[2]
References
- Firouzabadi, H.; Seddighi, M.; Ahmadi, Z. Arab; Sardarian, A. R. (1989). "Selective Oxidative Cleavage of Benzylic Carbon-Nitrogen Double Bonds Under Non-Aqueous Condition with Tetrakis(pyridine)-Silver Dichromate [(Py)2Ag]2Cr2O7". Synthetic Communications. 19 (19): 3385. doi:10.1080/00397918908052745.
- Firouzabadi, H.; Sardarian, A.; Gharibi, H. (1984). "Tetrakis (Pyridine)silver Dichromate Py4Ag2Cr207 - A Mild and Efficient Reagent for the Conversion of Benzylic and Allylic Alcohols to Their Corresponding Carbonyl Compounds". Synthetic Communications. 14: 89–94. doi:10.1080/00397918408060869.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.