Simple cell
A simple cell in the primary visual cortex is a cell that responds primarily to oriented edges and gratings (bars of particular orientations). These cells were discovered by Torsten Wiesel and David Hubel in the late 1950s.[1]
| Simple cell | |
|---|---|
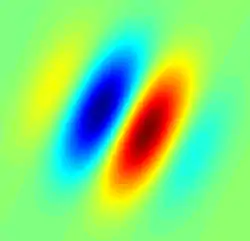 Gabor filter-type receptive field typical for a simple cell. Blue regions indicate inhibition, red facilitation | |
| Details | |
| Part of | primary visual cortex |
| System | Visual system |
| Anatomical terminology | |
Such cells are tuned to different frequencies and orientations, even with different phase relationships, possibly for extracting disparity (depth) information and to attribute depth to detected lines and edges.[2] This may result in a 3D 'wire-frame' representation as used in computer graphics. The fact that input from the left and right eyes is very close in the so-called cortical hypercolumns is an indication that depth processing occurs at a very early stage, aiding recognition of 3D objects.
Later, many other cells with specific functions have been discovered: (a) end-stopped cells which are thought to detect singularities like line and edge crossings, vertices and line endings; (b) bar and grating cells. The latter are not linear operators because a bar cell does not respond when seeing a bar which is part of a periodic grating, and a grating cell does not respond when seeing an isolated bar.
Using the mathematical Gabor model with sine and cosine components (phases), complex cells are then modeled by computing the modulus of complex Gabor responses. Both simple and complex cells are linear operators and are seen as filters because they respond selectively to a large number of patterns.
However, it has been claimed that the Gabor model does not conform to the anatomical structure of the visual system as it short-cuts the LGN and uses the 2D image as it is projected on the retina. Azzopardi and Petkov[3] have proposed a computational model of a simple cell, which combines the responses of model LGN cells with center-surround receptive fields (RFs). They call it Combination of RFs (CORF) model. Besides orientation selectivity, it exhibits cross orientation suppression, contrast invariant orientation tuning and response saturation. These properties are observed in real simple cells but are not possessed by the Gabor model. Using simulated reverse correlation they also demonstrate that the RF map of the CORF model can be divided into elongated excitatory and inhibitory regions typical of simple cells.
Lindeberg [4][5][6] has derived axiomatically determined models of simple cells in terms of directional derivatives of affine Gaussian kernels over the spatial domain in combination with temporal derivatives of either non-causal or time-causal scale-space kernels over the temporal domain and shown that this theory both leads to predictions about receptive fields with good qualitative agreement with the biological receptive field measurements performed by DeAngelis et al.[7][8] and guarantees good theoretical properties of the mathematical receptive field model, including covariance and invariance properties under natural image transformations.[9]
History
These cells were discovered by Torsten Wiesel and David Hubel in the late 1950s.[10]
Hubel and Wiesel named these cells "simple," as opposed to "complex cell", because they shared the following properties:[11]
- They have distinct excitatory and inhibitory regions.
- These regions follow the summation property.
- These regions have mutual antagonism - excitatory and inhibitory regions balance themselves out in diffuse lighting.
- It is possible to predict responses of moving stimuli given the map of excitatory and inhibitory regions.
Some other researchers such as Peter Bishop and Peter Schiller used different definitions for simple and complex cells.[12]
References
- D. H. Hubel and T. N. Wiesel Receptive Fields of Single Neurones in the Cat's Striate Cortex J. Physiol. pp. 574-591 (148) 1959
- Freeman, R. D.; DeAngelis, G. C.; Ohzawa, I. (1990-08-31). "Stereoscopic depth discrimination in the visual cortex: neurons ideally suited as disparity detectors". Science. 249 (4972): 1037–1041. Bibcode:1990Sci...249.1037O. CiteSeerX 10.1.1.473.8284. doi:10.1126/science.2396096. ISSN 1095-9203. PMID 2396096.
- G. Azzopardi and N. Petkov A CORF computational model that relies on LGN input outperforms the Gabor function model Biological Cybernetics, vol. 106(3), pp. 177-189, DOI: 10.1007/s00422-012-0486-6, 2012
- Lindeberg, T. Generalized Gaussian scale-space axiomatics comprising linear scale-space, affine scale-space and spatio-temporal scale-space, Journal of Mathematical Imaging and Vision 40(1): 36-81, 2011.
- T. Lindeberg "A computational theory of visual receptive fields", Biological Cybernetics 107(6): 589-635, 2013
- T. Lindeberg "Time-causal and time-recursive spatio-temporal receptive fields", Journal of Mathematical Imaging and Vision 55(1): 50-88, 2016.
- G. C. DeAngelis, I. Ohzawa and R. D. Freeman "Receptive field dynamics in the central visual pathways". Trends Neurosci. 18(10), 451–457, 1995.
- G. C. DeAngelis and A. Anzai "A modern view of the classical receptive field: linear and non-linear spatio-temporal processing by V1 neurons. In: Chalupa, L.M., Werner, J.S. (eds.) The Visual Neurosciences, vol. 1, pp. 704–719. MIT Press, Cambridge, 2004.
- T. Lindeberg "Invariance of visual operations at the level of receptive fields", PLOS ONE 8(7): e66990, pages 1-33, 2013
- D. H. Hubel and T. N. Wiesel Receptive Fields of Single Neurones in the Cat's Striate Cortex J. Physiol. pp. 574-591 (148) 1959
- D. H. Hubel and T. N. Wiesel Receptive Fields, Binocular Interaction and Functional Architecture in the Cat's Visual Cortex J. Physiol. 160 pp. 106-154 1962
- Brain and Visual Perception: The Story of a 25-Year Collaboration D. H. Hubel and T. N. Wiesel Oxford 2005
See also
- Visual system
- Spatiotemporal receptive field
- Axiomatic theory of receptive fields