Skatole
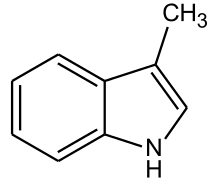
Skatole or 3-methylindole is an organic compound belonging to the indole family. It occurs naturally in the feces of mammals and birds and is the primary contributor to fecal odor. In low concentrations, it has a flowery smell and is found in several flowers and essential oils, including those of orange blossoms, jasmine, and Ziziphus mauritiana.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Methyl-1H-indole | |||
| Other names
3-Methylindole 4-Methyl-2,3-benzopyrrole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.338 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C9H9N | |||
| Molar mass | 131.178 g·mol−1 | ||
| Appearance | White crystalline solid | ||
| Odor | Feces | ||
| Melting point | 93 to 95 °C (199 to 203 °F; 366 to 368 K) | ||
| Boiling point | 265 °C (509 °F; 538 K) | ||
| Insoluble | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It is used as a fragrance and fixative in many perfumes and as an aroma compound. Its name derives from the Greek root skato-, meaning 'feces'. Skatole was discovered in 1877 by the German physician Ludwig Brieger (1849–1919).[1][2][3]
Original: Ich habe mich zuerst mit der Untersuchung der flüchtigen Bestandtheile der Excremente aus sauerer Lösung beschäftigt. Es wurden dabei die flüchtigen Fettsäuren: Essigsäure, normale und Isobuttersäure, sowie die aromatischen Substanzen: Phenol, Indol und eine neue dem Indol verwandte Substanz, die ich Skatol nennen werde, erhalten.
Translation: I was occupied initially with the investigation of the volitile components of excrement in acidic solution. One obtained thereby volitile fatty acids; acetic acid; normal and isobutyric acid; as well as the aromatic substances: phenol, indole and a new substance which is related to indole and which I will name "skatole". Das Skatol ... (von το σχατος = faeces) ... (Skatole ... (from το σχατος = feces) ....) —Brieger (1878), page 130:
Biosynthesis, chemical synthesis, and reactions
Skatole is derived from the amino acid tryptophan in the digestive tract of mammals. Tryptophan is converted to indoleacetic acid, which decarboxylates to give the methylindole.[4][5]
Skatole can be synthesized via the Fischer indole synthesis.[6]
It gives a violet color upon treatment with potassium ferrocyanide.
Insect attractant
Skatole is one of many compounds that are attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait for these bees for study.[7] It is also known for being an attractant for the Tasmanian grass grub beetle (Aphodius tasmaniae).[8]
Skatole has been shown to be an attractant to gravid mosquitoes in both field and laboratory conditions. Because this compound is present in feces, it is found in combined sewage overflows (CSO), as streams and lakes containing CSO water have untreated human and industrial waste. CSO sites are thus of particular interest when studying mosquito-borne diseases such as West Nile virus.[9]
Animal studies
Skatole occurs naturally in the feces of all species of mammals and birds, and in the bovine rumen.[10]
Skatole has been shown to cause pulmonary edema in goats, sheep, rats, and some strains of mice. It appears to selectively target club cells, which are the major site of cytochrome P450 enzymes in the lungs. These enzymes convert skatole to a reactive intermediate, 3-methyleneindolenine, which damages cells by forming protein adducts (see fog fever).[11]
With the testicular steroid androstenone, skatole is regarded as a principal determinant of boar taint.[12]
Skatole contributes to bad breath.[13]
Application
Skatole is the starting material in the synthesis of atiprosin.
See also
- 1-Methylindole
- 2-Methylindole (methylketol)
- 5-Methylindole
- 7-Methylindole
- Cadaverine
References
- Brieger, Ludwig (1877). "Über die flüchtigen Bestandtheile der menschlichen Excremente"" [On the volatile components of human excrement]. Berichte der Deutschen Chemischen Gesellschaft. 10: 1027–1032. doi:10.1002/cber.187701001288. Retrieved 3 November 2020.
- Brieger, Ludwig (1878). "Über die flüchtigen Bestandtheile der menschlichen Excremente". Journal für Praktische Chemie. 17: 124–138. doi:10.1002/prac.18780170111. Retrieved 3 November 2020.
Das Skatol ... (von το σχατος = faeces) ... (Skatole ... (from το σχατος = feces....)
- Brieger, Ludwig (1879). "Über Skatol" [On skatole]. Berichte der Deutschen Chemischen Gesellschaft. 12 (2): 1985–1988. doi:10.1002/cber.187901202206. Retrieved 3 November 2020.
- Whitehead, T. R.; Price, N. P.; Drake, H. L.; Cotta, M. A. (25 January 2008). "Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure". American Society for Microbiology:Applied and Environmental Microbiology. 74 (6): 1950–3. doi:10.1128/AEM.02458-07. PMC 2268313. PMID 18223109.
- Yokoyama, M. T.; Carlson, J. R. (1979). "Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole". The American Journal of Clinical Nutrition. 32 (1): 173–178. doi:10.1093/ajcn/32.1.173. PMID 367144.
- Emil Fischer (1886) "Indole aus Phenylhydrazin" (Indole from phenylhydrazine), Annalen der Chemie, vol. 236, pages 126-151; for Fischer's synthesis of skatole, see page 137. (Fischer was not the first to prepare skatole. It was prepared, via other methods, in 1880 by von Baeyer, and in 1883 by Otto Fischer and German and by Fileti.)
- Schiestl, F.P. & Roubik, D.W. (2004). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. hdl:20.500.11850/57276. PMID 12647866. S2CID 2845587.
- Osborne, G. O.; Penman, D. R.; Chapman, R. B. (1975). "Attraction of Aphodius tasmaniae Hope to skatole". Australian Journal of Agricultural Research. 26 (5): 839–841. doi:10.1071/AR9750839.
- Beechler, J W., J G Miller, and M S Mulla (1994). "Field evaluation of synthetic compounds mediating oviposition in Culex mosquitoes (Diptera: Culicidae)". J Chem Ecol. 20 (2): 281–291. doi:10.1007/BF02064436. PMID 24242053. S2CID 23784247.CS1 maint: multiple names: authors list (link)
- Yokoyama, M. T.; Carlson, J. R.; Holdeman, L. V. (1977). "Isolation and characteristics of a skatole-producing Lactobacillus sp. from the bovine rumen" (PDF). Applied and Environmental Microbiology. 34 (6): 837–842. doi:10.1128/AEM.34.6.837-842.1977. PMC 242757. PMID 563703. Retrieved 17 October 2020.
- Miller, M; Kottler, S; Ramos-Vara, J; Johnson, P; Ganjam, V; Evans, T (2003). "3-Methylindole Induces Transient Olfactory Mucosal Injury in Ponies". Veterinary Pathology. 40 (4): 363–70. doi:10.1354/vp.40-4-363. PMID 12824507.
- Wesoly, R.; Weiler, U. (2012). "Nutritional Influences on Skatole Formation and Skatole Metabolism in the Pig". Animals. 2 (2): 221–242. doi:10.3390/ani2020221. PMC 4494329. PMID 26486918.
- Franklin, Deborah (1 May 2013). "To Beat Bad Breath, Keep the Bacteria in Your Mouth Happy". Scientific American. Retrieved 3 November 2020.