Sodium bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula ((CH3)3Si)2NNa. This species, usually called NaHMDS (sodium hexamethyldisilazide), is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.[1]
amide.png.webp) | |
 | |
amide_trimer_from_crystal.png.webp) | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide | |
| Other names
Sodium hexamethyldisilazide Sodium hexamethyldisilazane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | NaHMDS |
| 3629917 | |
| ChemSpider | |
| ECHA InfoCard | 100.012.713 |
| EC Number |
|
PubChem CID |
|
| UN number | UN 3263 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H18NNaSi2 | |
| Molar mass | 183.37 g/mol |
| Appearance | off-white solid |
| Density | 0.9 g/cm3, solid |
| Melting point | 171 to 175 °C (340 to 347 °F; 444 to 448 K) |
| Boiling point | 170 °C (338 °F; 443 K) 2 mmHg |
| reacts with water | |
| Solubility in other solvents | THF, benzene toluene |
| Structure | |
| Triangular pyramidal | |
| Hazards | |
| Main hazards | Highly flammable, corrosive |
| R-phrases (outdated) | R11 R15 R34 |
| S-phrases (outdated) | S16 S24/25 |
| Related compounds | |
Other cations |
Lithium bis(trimethylsilyl)amide (LiHMDS) Potassium bis(trimethylsilyl)amide |
Related compounds |
Lithium diisopropylamide (LDA) KH |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
NaHMDS is quickly destroyed by water to form sodium hydroxide and bis(trimethylsilyl)amine.
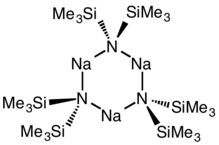
Structure
Although the N-Na bond is polar covalent as a solid, when dissolved in nonpolar solvents this compound is trimeric, consisting of a central Na3N3 ring.[2]
Applications in synthesis
NaHMDS is used as a base in organic synthesis. Typical reactions:
- To deprotonate ketones and esters to generate enolate derivatives.[3]
- Generate carbenes by dehydrohalogenation of halocarbons. These carbene reagents add to alkenes to give substituted cyclopropanes and cyclopropenes.[4]
- To deprotonation of phosphonium salts, generating Wittig reagents.
NaHMDS is also used as a base to deprotonate other compounds containing weakly acidic O-H, S-H, and N-H bonds. These include cyanohydrins and thiols.[5]
NaHMDS is reagent to convert alkyl halides to amines in a two step process that begins with N-alkylation followed by hydrolysis of the N-Si bonds.
- ((CH3)3Si)2NNa + RBr → ((CH3)3Si)2NR + NaBr
- ((CH3)3Si)2NR + H2O → ((CH3)3Si)2O + RNH2
This method has been extended to aminomethylation via the reagent (CH3)3Si)2NCH2OMe, which contains a displaceable methoxy group.
See also
References
- Watson, B. T.; Lebel, H. "Sodium bis(trimethylsilyl)amide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rs071m.pub2
- Driess, Matthias; Pritzkow, Hans; Skipinski, Markus; Winkler, Uwe (1997). "Synthesis and Solid State Structures of Sterically Congested Sodium and Cesium Silyl(fluorosilyl)phosphanide Aggregates and Structural Characterization of the Trimeric Sodium Bis(trimethylsilyl)amide". Organometallics. 16 (23): 5108–5112. doi:10.1021/om970444c.
- Sergey A. Kozmin, Shuwen He, and Viresh H. Rawal. "Preparation of (E)-1-Dimethylamino-3-tert-Butyldimethylsiloxy-1,3-Butadiene". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 301
- Paul Binger, Petra Wedemann, and Udo H. Brinker. "Cyclopropene: A New Simple Synthesis and its Diels-Alder Reaction with Cyclopentadiene". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 231
- J. Christopher McWilliams, Fred J. Fleitz, Nan Zheng, and Joseph D. Armstrong, III. "Preparation of n-Butyl 4-Chlorophenyl Sulfide". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 147