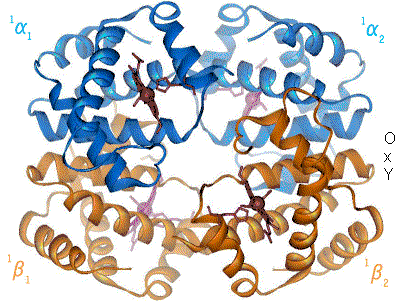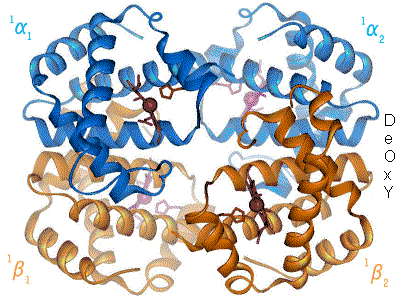
Structural biology
Structural biology is a branch of molecular biology, biochemistry, and biophysics concerned with the molecular structure of biological macromolecules (especially proteins, made up of amino acids, RNA or DNA, made up of nucleotides, and membranes, made up of lipids), how they acquire the structures they have, and how alterations in their structures affect their function.[1] This subject is of great interest to biologists because macromolecules carry out most of the functions of cells, and it is only by coiling into specific three-dimensional shapes that they are able to perform these functions. This architecture, the "tertiary structure" of molecules, depends in a complicated way on each molecule's basic composition, or "primary structure."
| Part of a series on |
| Biochemistry |
|---|
 |
| Key components |
| History of Biochemistry |
| Glossaries |
|
| Portals: Biochemistry |
Biomolecules are too small to see in detail even with the most advanced light microscopes. The methods that structural biologists use to determine their structures generally involve measurements on vast numbers of identical molecules at the same time. These methods include:
- Mass spectrometry
- Macromolecular crystallography
- Neutron diffraction
- Proteolysis
- Nuclear magnetic resonance spectroscopy of proteins (NMR)
- Electron paramagnetic resonance (EPR)
- Cryogenic Electron Microscopy (cryoEM)
- Electron crystallography and Microcrystal electron diffraction
- Multiangle light scattering
- Small angle scattering
- Ultrafast laser spectroscopy
- Dual-polarization interferometry and circular dichroism
Most often researchers use them to study the "native states" of macromolecules. But variations on these methods are also used to watch nascent or denatured molecules assume or reassume their native states. See protein folding.
A third approach that structural biologists take to understanding structure is bioinformatics to look for patterns among the diverse sequences that give rise to particular shapes. Researchers often can deduce aspects of the structure of integral membrane proteins based on the membrane topology predicted by hydrophobicity analysis. See protein structure prediction.
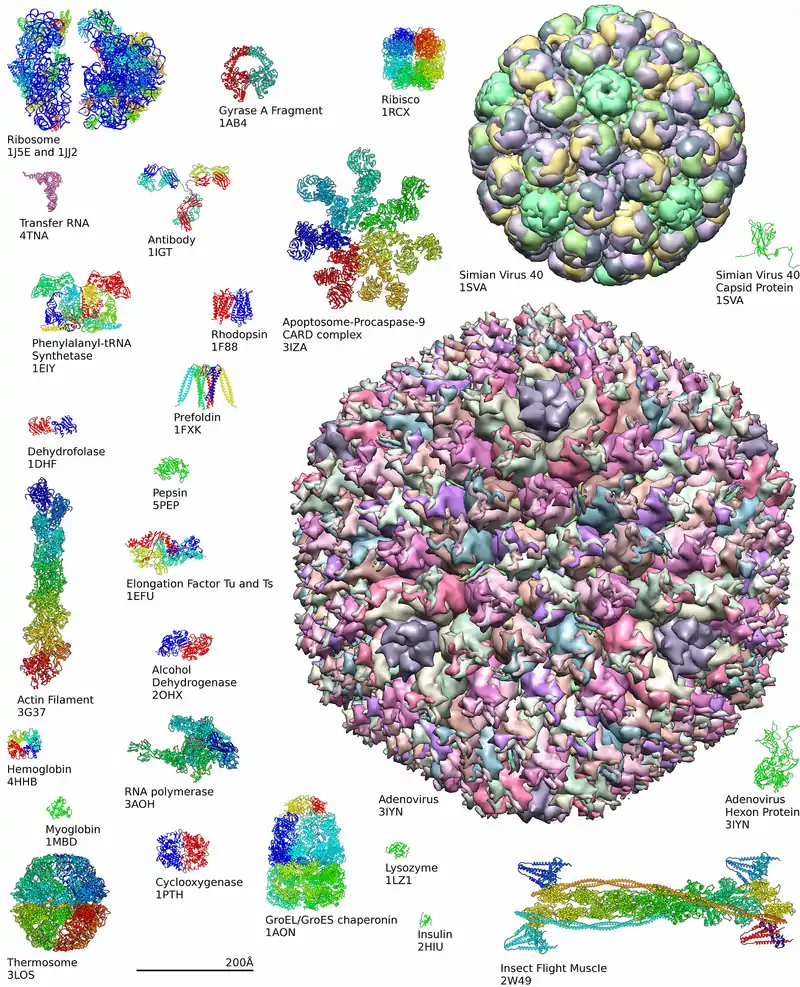
In the past few years it has become possible for highly accurate physical molecular models to complement the in silico study of biological structures. Examples of these models can be found in the Protein Data Bank.
Computational techniques like Molecular Dynamics simulations can be used in conjunction with empirical structure determination strategies to extend and study protein structure, conformation and function.[2]
See also
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
- Structural domain
- Structural motif
- Protein subunit
- Molecular model
- Cooperativity
- Chaperonin
- Structural genomics
- Stereochemistry
- Resolution (electron density)
- Proteopedia The collaborative, 3D encyclopedia of proteins and other molecules.
- Protein structure prediction
References
- Banaszak, Leonard J. (2000). Foundations of Structural Biology. Burlington: Elsevier. ISBN 9780080521848.
- Karplus, Martin; McCammon, J. Andrew (1 September 2002). "Molecular dynamics simulations of biomolecules". Nature Structural Biology. 9 (9): 646–652. doi:10.1038/nsb0902-646. PMID 12198485.
External links
| At Wikiversity, you can learn more and teach others about Structural biology at the Department of Structural biology |