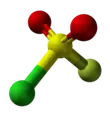
Sulfuryl chloride fluoride
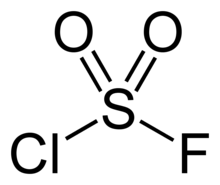
Sulfuryl chloride fluoride is the chemical compound with the formula SO2ClF. It is a colorless, easily condensed gas. It is a tetrahedral molecule.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sulfuryl chloride fluoride | |||
| Other names
Sulfuryl fluoride chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.737 | ||
PubChem CID |
|||
| RTECS number |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| ClFO2S | |||
| Molar mass | 118.52 g/mol | ||
| Appearance | colourless gas | ||
| Density | 1.623 g/cm3 at 0 °C | ||
| Melting point | −124.7 °C (−192.5 °F; 148.5 K) | ||
| Boiling point | 7.1 °C (44.8 °F; 280.2 K) | ||
| hydrolyses | |||
| Solubility in other solvents | SO2 and ether | ||
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| Main hazards | moderately toxic, corrosive | ||
| Safety data sheet | "External MSDS" | ||
| R-phrases (outdated) | R14 R34[1] | ||
| S-phrases (outdated) | S24/25 S26 S27 S28 S36/37/39 S38 S45[1] | ||
| Related compounds | |||
Related compounds |
SO2Cl2, SO2F2 ClSO2(NCO) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Liquified sulfuryl chloride fluoride is employed as a solvent for highly oxidizing compounds.[2]
Preparation
The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite:[3]
- SO2 + KF → KSO2F
This salt is then chlorinated to give sulfuryl chloride fluoride[4]
- KSO2F + Cl2 → SO2ClF + KCl
Further heating (180 °C) of potassium fluorosulfite with the sulfuryl chloride fluoride gives sulfuryl fluoride.
- KSO2F + SO2ClF → SO2F2 + KCl + SO2
Alternatively, sulfuryl chloride fluoride can be prepared without using gases as starting materials by treating sulfuryl chloride with ammonium fluoride or potassium fluoride in trifluoroacetic acid.[5]
- SO2Cl2 + NH4F → SO2ClF + NH4Cl
References
- "Sulfuryl chloride fluoride, distilled, 99%". Archived from the original on November 17, 2015. Retrieved February 19, 2013.
- Koppe, Karsten; Bilir, Vural; Frohn, Hermann-J.; Mercier, Hélène P. A.; Schrobilgen, Gary J. (2007). "Syntheses, Solution Multi-NMR Characterization, and Reactivities of [C6F5Xe]+Salts of Weakly Coordinating Borate Anions, [BY4]-(Y = CF3, C6F5, CN, or OTeF5)". Inorganic Chemistry. 46 (22): 9425–9437. doi:10.1021/ic7010138. PMID 17902647.
- Seel, F.; Czerepinski, Ralph G.; Cady, George H. (1967). Potassium Fluorosulfite. Inorg. Synth. Inorganic Syntheses. 9. pp. 113–115. doi:10.1002/9780470132401.ch29. ISBN 978-0-470-13240-1.
- Seel, F.; Duncan, Leonard C.; Czerepinski, Ralph G.; Cady, George H. (1967). Sulfuryl Chloride Fluoride and Sufluryl Fluoride. Inorg. Synth. Inorganic Syntheses. 9. pp. 111–113. doi:10.1002/9780470132401.ch28. ISBN 9780470132401.
- Prakash Reddy, V.; Bellew, Donald R.; Prakash, G. K. Surya (1992). "A Convenient Preparation of Sulfuryl Chloride Fluoride". Journal of Fluorine Chemistry. 56 (2): 195–197. doi:10.1016/S0022-1139(00)81102-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.