Surgery for benign prostatic hyperplasia
If medical treatment is not effective, surgery may need to be performed for benign prostatic hyperplasia.
| Surgery for benign prostatic hyperplasia | |
|---|---|
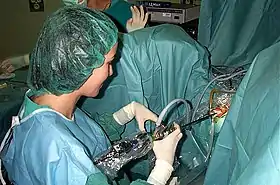 Transurethral resection of the prostate (TURP) |
Minimally invasive therapies
Minimally invasive therapies can offer faster recovery compared with traditional prostate surgery.[1]
Prostate laser surgery
Prostate laser surgery is used to relieve moderate to severe urinary symptoms caused by prostate enlargement. The surgeon inserts a scope through the penis tip into the urethra. A laser passed through the scope delivers energy to shrink or remove excess tissue that is preventing urine flow.[2]
Different types of prostate laser surgery include:
- VLAP technique involving the Nd:YAG laser with contact on the prostatic tissue.
- Photoselective Vaporisation of the Prostate (PVP). A laser is used to melt away (vaporize) excess prostate tissue and enlarge the urinary channel.[2] A high-power 180-watt 532 nm wavelength laser with a 650-micrometre laser fiber is used. This fiber has an internal reflection with a 70-degree deflecting angle. It is used to vaporize the tissue to the prostatic capsule. GreenLight 532 nm lasers target haemoglobin as the chromophore and typically have a penetration depth of 0.8 mm (twice as deep as holmium).
- Holmium Laser Ablation of the Prostate (HoLAP) is similar to PVP but uses a different type of laser.[2] HoLAP uses a 550 um disposable side-firing fiber that directs the beam from a high-power 100-watt laser at a 70-degree angle from the fiber axis. The holmium wavelength is 2,140 nm, which falls within the infrared portion of the spectrum and is invisible to the naked eye. Whereas GreenLight relies on haemoglobin as a chromophore, water within the target tissue is the chromophore for Holmium lasers. The penetration depth of Holmium lasers is <0.4 mm, avoiding complications associated with tissue necrosis often found with the deeper penetration and lower peak powers of Nd:YAG lasers used in the 1990s.[3]
- * Holmium Laser Enucleation of the Prostate (HoLEP) is used to cut and remove the excess tissue that is blocking the urethra. Another instrument is then used to cut the prostate tissue into small pieces that are easily removed. HoLEP can be an option for men who have a severely enlarged prostate and is the only prostate size-independent treatment option approved by the American Urologic Association.[4] HoLEP is largely similar to the HoLAP procedure; the main difference is that instead of ablating the tissue, the laser cuts a portion of the prostate, which is then cut into smaller pieces and flushed with irrigation fluid. As with the HoLAP procedure, there is little bleeding during or after the procedure. Three 2015 reviews found that HoLEP is superior to TURP in some respects and for some patients.[3][5][6]
Both wavelengths, GreenLight and Holmium, ablate approximately one to two grams of tissue per minute.
Post-surgical care often involves placement of a Foley catheter or a temporary prostatic stent to permit healing and allow urine to drain from the bladder.
Non-laser treatments
The two most common types of office-based therapies are transurethral microwave thermotherapy (TUMT) and transurethral needle ablation (TUNA). Both rely on delivering enough energy to create sufficient heat to cause cell death (necrosis) in the prostate. The goal is to cause enough necrosis so that, when the dead tissue is reabsorbed by the body, the prostate shrinks, relieving the obstruction of the urethra. These procedures are typically performed with local anesthesia, and the patient returns home the same day. Some urologists have studied and published long-term data on the outcomes of these procedures, with data out to five years.
- Transurethral microwave thermotherapy (TUMT) was originally approved by the United States Food and Drug Administration (FDA) in 1996, with the first generation system by EDAP Technomed. Since 1996, other companies have received FDA approval for TUMT devices, including Urologix, Dornier, Thermatrix, Celsion, and Prostalund. Multiple clinical studies have been published on TUMT. The general principle underlying all the devices is that a microwave antenna that resides in a urethral catheter is placed in the intraprostatic area of the urethra. The catheter is connected to a control box outside of the patient's body and is energized to emit microwave radiation into the prostate to heat the tissue and cause necrosis. It is a one-time treatment that takes approximately 30 minutes to 1 hour, depending on the system used. It takes approximately 4 to 6 weeks for the damaged tissue to be reabsorbed into the patient's body. Some of the devices incorporate circulating coolant through the treatment area with the intent of preserving the urethra while the microwave energy heats the prostatic tissue surrounding the urethra.
- Transurethral needle ablation (TUNA) operates with a different type of energy, radio frequency (RF) energy, but is designed along the same premise as TUMT devices, that the heat the device generates will cause necrosis of the prostatic tissue and shrink the prostate. The TUNA device is inserted into the urethra using a rigid scope much like a cystoscope. The energy is delivered into the prostate using two needles that emerge from the sides of the device, through the urethral wall and into the prostate. The needle-based ablation devices are very effective at heating a localized area to a high enough temperature to cause necrosis. The treatment is typically performed in one session, but may require multiple sticks of the needles depending on the size of the prostate. The most recent American Urological Association (AUA) Guidelines for the Treatment of BPH from 2018 stated that "TUNA is not recommended for the treatment of LUTS/BPH".[7]
The American Urological Association (AUA) guidelines for the treatment of BPH from 2018 list minimally invasive therapies including TUMT - but not TUNA - as acceptable alternatives for certain patients with BPH.[7]
However, the European Association of Urology (EAU) has - as of 2019 - removed both TUMT and TUNA from its guidelines.[8]
Invasive therapies
The two invasive surgical procedures done for BPH:
- Transurethral resection of the prostate (TURP): In general prior to emergence of laser technologies, TURP had been considered the gold standard of prostate interventions for people who require a procedure. This involves removing (part of) the prostate by inserting a resectoscope (cystoscope) through the urethra. However, after this endoscopic surgery the ejaculations are dry in about 65% of patients, unless a novel, ejaculation preserving, altered technique of TURP is applied.[9][10]
- Simple prostatectomy can also be offered to men who have large prostates (>50 grams). This can be done by open technique, laparoscopically, or with robotic assistance.[11]
Efforts to find newer surgical methods have resulted in newer approaches and different types of energies being used to treat the enlarged gland. However some of the newer methods for reducing the size of an enlarged prostate, have not been around long enough to fully establish their safety or side-effects. These include various methods to destroy or remove part of the excess tissue while trying to avoid damaging what remains. Transurethral electrovaporization of the prostate (TVP), laser TURP, visual laser ablation (VLAP), ethanol injection, and others are studied as alternatives.[3]
Complications of Prostate Surgery
The two most feared complications of prostate surgery are erectile dysfunction and stress urinary incontinence.[12] The type of complications depend on the treatment modality used:
- Urinary incontinence can happen after prostate surgery, especially stress urinary incontinence. The prostate is located right beneath the bladder, and surrounds the urethral sphincter. Any damage to the sphincter or surrounding muscles and nerves can lead to urinary incontinence. The problem is most severe in the first 6 to 12 months after treatment, but usually resolves on its own within this time.[13] If the problem persists, conservative management is the first line treatment. This includes lifestyle modifications, kegel exercises, and bladder training.[14][15] Artificial urinary sphincter implantation is considered the gold standard in moderate to severe cases if conservative management fails.[16]
- About 50% of patients who undergo prostatectomy will have some degree of erectile dysfunction. Treatment options include the use of oral drugs, vacuum devices, or a penile implant.[17]
Other
The National Institute for Health and Care Excellence (NICE) of the UK in 2018 classified some novel methods as follows.[18]
Recommended:
- Urolift System[19]
- Aquablation therapy[20]
- Prostate steam treatment[21]
Not recommended:
General prospects of surgery success
The success of surgery for benign prostatic hyperplasia (BPH) – as measured by a significant reduction of lower urinary tract symptoms (LUTS) – strongly depends on a reliable (unequivocal) pre-surgery diagnosis of bladder outlet obstruction (BOO). A pre-surgery diagnosis of other LUTS only, such as overactive bladder (OAB) with or without urinary incontinence predicts little or no success after surgery.[23]
If BOO is present or not can be determined by reliable non-invasive tests, such as the Penile cuff test (PCT). In this test, first published in 1997, a software-steered inflatable cuff (similar as in a blood pressure meter) is placed around the penis to measure the pressure of urinary flow.[24] By applying this methode, a study of 2013 showed that 94% of the patients with the pre-surgery test result "Obstruction" had a successful surgery outcome. In contrast, 70% of the patients with the pre-surgery test result "No Obstruction" had a non-successful surgery outcome.[25][23]
If BPH with obstruction additionally presents with overactive bladder (OAB), which is the case in about 50% of patients,[26] this latter symptom (OAB) persits even post-surgery in about 20% of patients. However, this rate only applies to a period of a few years. 10–15 years after surgery 48 of 55 patients (87%) with obstruction and OAB had kept their post-surgery reduction of obstruction, but their OAB symptoms had gone back to the pre-surgery status.[27]
References
- "Laser PVP surgery" mayocilic.org
- "Prostate laser surgery" mayoclinic.org
- Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Gilling P, Gratzke C (2015). "A Systematic Review and Meta-analysis of Functional Outcomes and Complications Following Transurethral Procedures for Lower Urinary Tract Symptoms Resulting from Benign Prostatic Obstruction: An Update" (PDF). Eur Urol. 67 (6): 1066–1096. doi:10.1016/j.eururo.2014.06.017. PMID 24972732.CS1 maint: multiple names: authors list (link) Comment in same issue of the journal.
- Parsons, J. Kellogg; Dahm, Philipp; Köhler, Tobias S.; Lerner, Lori B.; Wilt, Timothy J. (2020). "Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline Amendment 2020". Journal of Urology. 204 (4): 799–804. doi:10.1097/JU.0000000000001298. ISSN 0022-5347. PMID 32698710.
- van Rij S, Gilling P (2015). "Recent advances in treatment for Benign Prostatic Hyperplasia". F1000Res. 4: 1482. doi:10.12688/f1000research.7063.1. PMC 4754003. PMID 26918132.
- Michalak, J; Tzou, D; Funk, J (2015). "HoLEP: the gold standard for the surgical management of BPH in the 21(st) Century". American Journal of Clinical and Experimental Urology. 3 (1): 36–42. PMC 4446381. PMID 26069886.
- Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS (2018). "Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline". J Urol. 200 (3): 612–619. doi:10.1016/j.juro.2018.05.048. PMID 29775639.CS1 maint: multiple names: authors list (link)
- EAU: Management of Non-neurogenic Male LUTS - Summary of Changes 2019.
- Lebdai S, Chevrot A, Doizi S, Pradere B, Delongchamps NB, Benchikh A (2019). "Do patients have to choose between ejaculation and miction? A systematic review about ejaculation preservation technics for benign prostatic obstruction surgical treatment". World J Urol. 37 (2): 299–308. doi:10.1007/s00345-018-2368-6. PMID 29967947. S2CID 49556196.CS1 maint: multiple names: authors list (link)
- Marien T, Kadihasanoglu M, Miller NL (2016). "Holmium laser enucleation of the prostate: patient selection and perspectives". Res Rep Urol. 8: 181–192. doi:10.2147/RRU.S100245. PMC 5085273. PMID 27800470.CS1 maint: multiple names: authors list (link)
- Helfand, Brian; Mouli, Samdeep; Dedhia, Raj; McVary, Kevin T. (December 2006). "Management of lower urinary tract symptoms secondary to benign prostatic hyperplasia with open prostatectomy: results of a contemporary series". The Journal of Urology. 176 (6 Pt 1): 2557–2561, discussion 2561. doi:10.1016/j.juro.2006.07.143. ISSN 0022-5347. PMID 17085158.
- Michaelson, M. Dror; Cotter, Shane E.; Gargollo, Patricio C.; Zietman, Anthony L.; Dahl, Douglas M.; Smith, Matthew R. (2008). "Management of Complications of Prostate Cancer Treatment". CA: A Cancer Journal for Clinicians. 58 (4): 196–213. doi:10.3322/CA.2008.0002. ISSN 1542-4863. PMC 2900775. PMID 18502900.
- "Urinary Incontinence After Prostate Surgery: Everything You Need To Know". URINARY INCONTINENCE EDUCATION | BLADDER HEALTH | NATIONAL ASSOCIATION FOR CONTINENCE. Retrieved 2020-04-16.
- Anderson, Coral A; Omar, Muhammad Imran; Campbell, Susan E; Hunter, Kathleen F; Cody, June D; Glazener, Cathryn MA (2015-01-20). "Conservative management for postprostatectomy urinary incontinence". Cochrane Database of Systematic Reviews. 1: CD001843. doi:10.1002/14651858.cd001843.pub5. ISSN 1465-1858. PMC 7025637. PMID 25602133.
- Anderson, Coral A.; Omar, Muhammad Imran; Campbell, Susan E.; Hunter, Kathleen F.; Cody, June D.; Glazener, Cathryn M. A. (2015-01-20). "Conservative management for postprostatectomy urinary incontinence". The Cochrane Database of Systematic Reviews. 1: CD001843. doi:10.1002/14651858.CD001843.pub5. ISSN 1469-493X. PMC 7025637. PMID 25602133.
- Moore, Katie C.; Lucas, Malcolm G. (2010). "Management of male urinary incontinence". Indian Journal of Urology. 26 (2): 236–244. doi:10.4103/0970-1591.65398. ISSN 0970-1591. PMC 2938549. PMID 20877603.
- "Erectile Dysfunction After Prostate Cancer". www.hopkinsmedicine.org. Retrieved 2020-04-16.
- National Institute for Health and Care Excellence (NICE): Current care pathway (for BPH), August 2018.
- Ray A, Morgan H, Wilkes A, Carter K, Carolan-Rees G (2016). "The Urolift System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A NICE Medical Technology Guidance". Appl Health Econ Health Policy. 14 (5): 515–26. doi:10.1007/s40258-015-0218-x. PMC 5025508. PMID 26832146.CS1 maint: multiple names: authors list (link)
- National Institute for Health and Care Excellence (NICE): Transurethral water jet ablation for lower urinary tract symptoms caused by benign prostatic hyperplasia, Interventional procedures guidance, 19 September 2018.
- National Institute for Health and Care Excellence (NICE): Rezum for treating benign prostatic hyperplasia, Medtech innovation briefing, 24 August 2018.
- National Institute for Health and Care Excellence (NICE): Current care pathway (for BPH), issue "Minimally invasive treatments", August 2018.
- Jiang YH, Kuo HC (2017). "Recent research on the role of urodynamic study in the diagnosis and treatment of male lower urinary tract symptoms and urinary incontinence". Tzu Chi Medical Journal. 29 (2): 72–78. doi:10.4103/tcmj.tcmj_19_17 (inactive 2021-01-16). PMC 5509199. PMID 28757770.CS1 maint: DOI inactive as of January 2021 (link)
- Malde S, Nambiar AK, Umbach R, Lam TB, Bach T, Bachmann A, Drake MJ, Gacci M, Gratzke C, Madersbacher S, Mamoulakis C, Tikkinen KA, Gravas S (2017). "Systematic Review of the Performance of Noninvasive Tests in Diagnosing Bladder Outlet Obstruction in Men with Lower Urinary Tract Symptoms" (PDF). Eur Urol. 71 (3): 391–402. doi:10.1016/j.eururo.2016.09.026. hdl:10138/233866. PMID 27687821.CS1 maint: multiple names: authors list (link)
- Losco G, Keedle L, King Q (2013). "Non-invasive urodynamics predicts outcome prior to surgery for prostatic obstruction". BJU Int. 112 Suppl 2: 61–4. doi:10.1111/bju.12382. PMID 24127677. S2CID 46245863.CS1 maint: multiple names: authors list (link)
- Eapen RS, Radomski SB (2016). "Review of the epidemiology of overactive bladder". Res Rep Urol. 8: 71–6. doi:10.2147/RRU.S102441. PMC 4902138. PMID 27350947.
- Thomas AW, Abrams P (2000). "Lower urinary tract symptoms, benign prostatic obstruction and the overactive bladder". BJU Int. 85 Suppl 3: 57–68, discussion 70–1. doi:10.1111/j.1464-410X.2000.tb16953.x. PMID 11954200. S2CID 42369935.