High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU) is a non-invasive therapeutic technique[1] that uses non-ionizing ultrasonic waves to heat or ablate tissue. HIFU can be used to increase the flow of blood or lymph, or to destroy tissue, such as tumors, via thermal and mechanical mechanisms. Given the prevalence and relatively low cost of ultrasound, HIFU has been subject to much research and development. The premise of HIFU is that it is a non-invasive low cost therapy that can at minimum outperform the current standard of care.
| High-intensity focused ultrasound | |
|---|---|
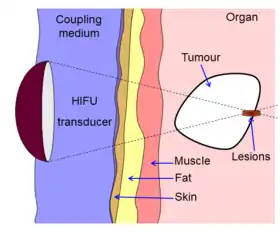 Diagram showing how HIFU can be used to destroy tissue in the body. An acoustic lens is used to focus sound to a small point in the body. The sound propagates through many layers of tissue. Because of the focal gain, only tissue at the focus is destroyed. | |
| Other names | Magnetic resonance guided focused ultrasound surgery (MRgFUS), Focused Ultrasound Surgery (FUS) |
The technology is similar to ultrasonic imaging, although lower frequencies and continuous, rather than pulsed waves are used to achieve the necessary thermal doses. However, pulsed waves may also be used if mechanical rather than thermal damage is desired. Acoustic lenses are often used to achieve the necessary intensity at the target tissue without damaging the surrounding tissue. An analogy is using a magnifying glass to focus sunlight; only the focal point of the magnifying glass has high intensity. Although lenses have traditionally been used, phased arrays are increasingly common as they allow the focal position to be easily changed.
HIFU is traditionally combined with other imaging techniques such as medical ultrasound or MRI to enable guidance of the treatment and monitoring.
History
The first investigations of HIFU for non-invasive ablation were reported by Lynn et al. in the early 1940s. Extensive important early work was performed in the 1950s and 1960s by William Fry and Francis Fry at the University of Illinois[2] and Carl Townsend, Howard White and George Gardner at the Interscience Research Institute of Champaign, Ill., culminating in clinical treatments of neurological disorders. In particular High Intensity ultrasound and ultrasound visualization was accomplished stereotaxically with a Cincinnati precision milling machine to perform accurate ablation of brain tumors. Until recently, clinical trials of HIFU for ablation were few (although significant work in hyperthermia was performed with ultrasonic heating), perhaps due to the complexity of the treatments and the difficulty of targeting the beam noninvasively. With recent advances in medical imaging and ultrasound technology, interest in HIFU ablation of tumors has increased.
The first commercial HIFU machine, called the Sonablate 200, was developed by the American company Focus Surgery, Inc. (Milipitas, CA) and launched in Europe in 1994 after receiving CE approval, bringing a first medical validation of the technology for benign prostatic hyperplasia (BPH). Comprehensive studies by practitioners at more than one site using the device demonstrated clinical efficacy for the destruction of prostatic tissue without loss of blood or long term side effects. Later studies on localized prostate cancer by Murat and colleagues at the Edouard Herriot Hospital in Lyon in 2006 showed that after treatment with the Ablatherm (EDAP TMS, Lyon, France), progression-free survival rates are very high for low- and intermediate- risk patients with recurrent prostate cancer (70% and 50% respectively)[3] HIFU treatment of prostate cancer is currently an approved therapy in Europe, Canada, South Korea, Australia, and elsewhere. As of 2012, clinical trials for the Sonablate 500 in the United States are ongoing for prostate cancer patients and those who have experienced radiation failure.[4]
Use of magnetic resonance-guided focused ultrasound was first cited and patented in 1992.[5][6] The technology was later transferred to InsighTec in Haifa Israel in 1998. The InsighTec ExAblate 2000 was the first MRgFUS system to obtain FDA market approval[7] in the United States.
Medical uses
There is no clear consensus on the boundaries between HIFU and other forms of therapeutic ultrasound. In academic literature, HIFU usually refers to the high levels of energy required to destroy tissue through ablation or cavitation, although it is also sometimes used to describe lower intensity applications such as occupational therapy and physical therapy.
Either way, HIFU is used to non-invasively heat tissue deep in the body without the need for an incision.[1] The main applications are the destruction of tissue, increasing perfusion and physical therapy. The use of ultrasound in the treatment of musculoskeletal conditions is another use in the physiotherapy setting.[8]
Neurological disorders
One of the first applications of HIFU was the treatment of Parkinson's disease in the 1940s. Although ineffective at the time, HIFU has the capacity to lesion pathology. A focused ultrasound system is approved in Israel, Canada, Europe, Korea and Russia to treat essential tremor,[9] neuropathic pain,[10] and Parkinsonian tremor.[11] This approach enables treatment of the brain without an incision or radiation. In 2016, the US Food and Drug Administration (FDA) approved Insightec's Exablate system to treat essential tremor.[12] Treatment for other thalamocortical dysrhythmias and psychiatric conditions are under investigation.[13]
Uterine adenomyosis and fibroids
Treatment for symptomatic uterine fibroids became the first approved application of HIFU by the US Food and Drug Administration (FDA) in October 2004.[7] Studies have shown that HIFU is safe and effective, and that patients have sustained symptomatic relief is sustained for at least two years without the risk of complications involved in surgery or other more invasive approaches.[14] Up to 16-20% of patients will require additional treatment.[15]
Cancers
HIFU is an attractive option for tumors in locations that are hard to reach or non-resectable.[16] Of particular interest are intestinal cancers and brain cancer. When recommending treatment, a clinician must weigh
Prostate Cancer
HIFU is being studied in men with prostate cancer.[17][18] HIFU was approved in the United States for prostate tissue ablation in 2015.[19][20][21] HIFU is also being used for ablation of prostate cancer.[22][23][24]
Liver Cancer
HIFU is well studied in liver cancer and in many studies report a high response rate and positive patient outcome.[25]
Abscopal Effect
During the treatment of metastasized liver cancer with HIFU, immune responses have been observed in locations that are distant from the focal region.[26] Although the mechanism of this systemic response is unknown, it is thought to be elicited by the release of tumor antigens with retained immunogenicity through histotripsy.[27]
Other cancers
HIFU has been successfully applied in treatment of cancer to destroy solid tumors of the bone, brain, breast, pancreas, rectum, kidney, testes, prostate.[28]
Palliative care
HIFU has been found to have palliative effects. CE approval has been given for palliative treatment of bone metastasis.[29] Experimentally, a palliative effect was found in cases of advanced pancreatic cancer.[30]
Prostate enlargement
Treatment of prostate enlargement (benign prostatic hyperplasia) by HIFU from inside the intestine (transrectal) has turned out to be unsuccessful.[31][32]
In some countries, not in USA, HIFU has also been offered from the inside of the prostate, that is, via a catheter in the prostatic urethra. Evidence as of 2019 is lacking.[33]
In England the National Institute for Health and Care Excellence (NICE) in 2018 classified the method as "not recommended".[34] In the US (as of 2019) not even the technical device needed for the treatment has been approved.[35]
Mechanism
HIFU beams are precisely focused on a small region of diseased tissue to locally deposit high levels of energy.
The focusing effect of the transducer allows high sound pressures to be delivered to a focal point without causing unwanted damage to other tissue. This increase in pressure can cause a number of effects including heating and cavitation.

- Ultrasound sources may be used to generate regional heating and mechanical changes in biological tissue, e.g. in and cancer treatment.
- Focused ultrasound may be used to generate highly localized heating to treat cysts and tumors (benign or malignant). This is known as Magnetic Resonance guided Focused Ultrasound (MRgFUS) or High Intensity Focused Ultrasound (HIFU). These procedures generally use lower frequencies than medical diagnostic ultrasound (from 0.250 to 2 MHz), but significantly higher energies. HIFU treatment is often guided by MRI.
- Focused ultrasound may be used to break up kidney stones by lithotripsy.
- Ultrasound may be used for cataract treatment by phacoemulsification.
In 2015 the FDA authorized two HIFU devices for the ablation of prostate tissue.[36]
Temperature
The temperature of tissue at the focus will rise to between 65 and 85 °C, destroying the diseased tissue by coagulative necrosis. If tissue is elevated above the threshold of 60 °C for longer than 1 second this process is irreversible.[37] Higher temperatures are usually avoided to prevent boiling of liquids inside the tissue. Each sonication (individual ultrasound energy deposition) treats a precisely defined portion of the targeted tissue. The entire therapeutic target is treated by using multiple sonications to create a volume of treated tissue, according to a protocol developed by the physician. Anesthesia is not required, but sedation is generally recommended.[38]
The amount of damage caused in the tissue can be modeled using Cumulative Equivalent Minutes (CEM). Several formulations of the CEM equation have been suggested over the years, but the equation currently in use for most research done in HIFU therapy comes from a 1984 paper by Dewey and Sapareto:[39]
with the integral being over the treatment time, R=0.5 for temperatures over 43 °C and 0.25 for temperatures between 43 °C and 37 °C, a reference temperature of 43 °C, and time in minutes. This formula is an empirical formula derived from experiments performed by Dewey and Sapareto by measuring the survival of cell cultures after exposure to heat.[40]
As an acoustic wave propagates through the tissue, part of it is absorbed and converted to heat. With focused beams, a very small region of heating can be achieved deep in tissues (usually on the order of millimeters). Tissue damage occurs as a function of both the temperature to which the tissue is heated and how long the tissue is exposed to this heat level in a metric referred to as "thermal dose". By focusing at more than one place or by scanning the focus, a volume can be thermally ablated.[41][42][43] Thermal doses of 120-240 min at 43 °C coagulate cellular protein and leads to irreversible tissue destruction.
There is some evidence that HIFU can be applied to cancers to disrupt the tumor microenvironment and trigger an immune response, as well as possibly enhance the efficacy of immunotherapy.[44][45]
Intertial Cavitation
At high enough acoustic intensities, cavitation (microbubbles forming and interacting with the ultrasound field) can occur. Microbubbles produced in the field oscillate and grow (due to factors including rectified diffusion), and can eventually implode (inertial or transient cavitation). During inertial cavitation, very high temperatures occur inside the bubbles, and the collapse during the rarefaction phase is associated with a shock wave and jets that can mechanically damage tissue.[46]
Stable Cavitation
Stable cavitation creates microstreaming which induces high shear forces on forces and leads to apoptosis. Elaborating, bubbles produced by the vaporization of water due to acoustic forces oscillate under a low-pressure acoustic field. Strong streaming may cause cell damage but also reduces tissue temperature via convective heat loss.[47]
Theory
There are several ways to focus ultrasound—via a lens (for example, a polystyrene lens), a curved transducer, a phased array, or any combination of the three. This concentrates it into a small focal zone; it is similar in concept to focusing light through a magnifying glass. This can be determined using an exponential model of ultrasound attenuation. The ultrasound intensity profile is bounded by an exponentially decreasing function where the decrease in ultrasound is a function of distance traveled through tissue:
is the initial intensity of the beam, is the attenuation coefficient (in units of inverse length), and z is distance traveled through the attenuating medium (e.g. tissue).
In this model, [48] is a measure of the power density of the heat absorbed from the ultrasound field. Sometimes, SAR is also used to express the amount of heat absorbed by a specific medium, and is obtained by dividing Q by the tissue density. This demonstrates that tissue heating is proportional to intensity, and that intensity is inversely proportional to the area over which an ultrasound beam is spread—therefore, focusing the beam into a sharp point (i.e. increasing the beam intensity) creates a rapid temperature rise at the focus.
The ultrasound beam can be focused in these ways:
- Geometrically, for example with a lens or with a spherically curved transducer.
- Electronically, by adjusting the relative phases of elements in an array of transducers (a "phased array"). By dynamically adjusting the electronic signals to the elements of a phased array, the beam can be steered to different locations, and aberrations in the ultrasound beam due to tissue structures can be corrected.
Beam Delivery
Beam delivery consists of beam steering and image guidance. The beam has the ability to pass through overlying tissues without harm and focus on a localized area with size limit of 3–4 cm.[49] The area at the focal point of the beam undergoes coagulative necrosis. Following ablation a distinct boundary forms between healthy and necrotic tissue (width less than 50 microns).[49]
Beam Steering
The most common transducer used is a concave focusing transducer with a fixed aperture and a fixed focal length.[49] Phased array transducers can also be used with different arrangements (flat/bowl).[49]
Image Guidance
HIFU therapy requires careful monitoring and so is usually performed in conjunction with other imaging techniques.
Pre-operative imaging, for instance CT and MRI, are usually used to identify general parameters of the target anatomy. Real-time imaging, on the other hand, is necessary for safe and accurate noninvasive targeting and therapy monitoring. Both MRI and Medical ultrasound imaging have been used for guidance in FUS treatment. These techniques are known as Magnetic Resonance guided Focused Ultrasound Surgery (MRgFUS)[50] and Ultrasound guided Focused Ultrasound Surgery (USgFUS) respectively.[1][51] MRgFUS is a 3D imaging technique which features high soft tissue contrast and provides information about temperature, thus allowing to monitor ablation. However, low frame rate makes this technique perform poorly in real-time imaging and high costs represent a significant limitation to its use.[52] USgFUS, differently, is a 2D imaging technique in which, although no system to provide quantitative information on temperature has been commercially developed so far, several benefits are exploited, such as high frame rate (up to 1000 images per second), low cost and minimal adverse health effects. Another reason why ultrasound is ideal for image guidance is it verifies the acoustic window in real time since it is the same modality as the therapy.[53] The implication of this is that if the target region is not visualized by ultrasound imaging before and during HIFU therapy, then it is unlikely that HIFU therapy will be effective in that specific region.[53] In addition, treatment outcomes can be estimated in real time through visual inspection of hyperechoic changes in standard B-mode images.[2]
References
- Dubinsky, Theodore J.; Cuevas, Carlos; Dighe, Manjiri K.; Kolokythas, Orpheus; Hwang, Joo Ha (2008). "High-Intensity Focused Ultrasound: Current Potential and Oncologic Applications". American Journal of Roentgenology. 190 (1): 191–199. doi:10.2214/AJR.07.2671. ISSN 0361-803X. PMID 18094311.
- Ebbini, Emad S.; Ter Haar, Gail (2015). "Ultrasound-guided therapeutic focused ultrasound: Current status and future directions". International Journal of Hyperthermia. 31 (2): 77–89. doi:10.3109/02656736.2014.995238. ISSN 0265-6736. PMID 25614047. S2CID 23590340.
- Gelet, A; Murat, François-Joseph; Poissonier, L (2007). "Recurrent Prostate Cancer After Radiotherapy – Salvage Treatment by High-intensity Focused Ultrasound". European Oncological Disease. 1 (1): 60–2.
- USHIFU (2012). "Clinical Information about HIFU in the U.S". Archived from the original on August 7, 2009.
- Hynynen, K.; Damianou, C.; Darkazanli, A.; Unger, E.; Levy, M.; Schenck, J. F. (1992). "On-line MRI monitored noninvasive ultrasound surgery". Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society: 350–351. doi:10.1109/IEMBS.1992.5760999. ISBN 978-0-7803-0785-8. S2CID 31185306.
- US 5247935, "Magnetic resonance guided focussed ultrasound surgery", issued March 19, 1992
- Food and Drug Administration Approval, ExAblate® 2000 System – P040003
- Robertson, VJ; Baker, KG (2001). "A review of therapeutic ultrasound: Effectiveness studies". Physical Therapy. 81 (7): 1339–50. doi:10.1093/ptj/81.7.1339. PMID 11444997.
- Elias, W. Jeffrey; Huss, Diane; Voss, Tiffini; Loomba, Johanna; Khaled, Mohamad; Zadicario, Eyal; Frysinger, Robert C.; Sperling, Scott A.; Wylie, Scott; Monteith, Stephen J.; Druzgal, Jason; Shah, Binit B.; Harrison, Madaline; Wintermark, Max (2013). "A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor". New England Journal of Medicine. 369 (7): 640–8. doi:10.1056/NEJMoa1300962. PMID 23944301.
- Jeanmonod, Daniel; Werner, Beat; Morel, Anne; Michels, Lars; Zadicario, Eyal; Schiff, Gilat; Martin, Ernst (2012). "Transcranial magnetic resonance imaging–guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain" (PDF). Neurosurgical Focus. 32 (1): E1. doi:10.3171/2011.10.FOCUS11248. PMID 22208894.
- Magara, Anouk; Bühler, Robert; Moser, David; Kowalski, Milek; Pourtehrani, Payam; Jeanmonod, Daniel (2014). "First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease". Journal of Therapeutic Ultrasound. 2: 11. doi:10.1186/2050-5736-2-11. PMC 4266014. PMID 25512869.
- FDA News Release. "FDA approves first MRI-guided focused ultrasound device to treat essential tremor", FDA, July 11, 2016
- Martin-Fiori, E (2014). Intraoperative Imaging and Image-Guided Therapy. New York: Springer. doi:10.1007/978-1-4614-7657-3_45. ISBN 978-1-4614-7657-3.
- Fennessy, Fiona; Fischer, Krisztina; McDannold, Nathan; Jolesz, Ferenc; Tempany, Clare (2015). "Potential of minimally invasive procedures in the treatment of uterine fibroids: a focus on magnetic resonance-guided focused ultrasound therapy". International Journal of Women's Health. 7: 901–12. doi:10.2147/IJWH.S55564. PMC 4654554. PMID 26622192.
- Stewart, Elizabeth A.; Gostout, Bobbie; Rabinovici, Jaron; Kim, Hyun S.; Regan, Lesley; Tempany, Clare M. C. (2007). "Sustained Relief of Leiomyoma Symptoms by Using Focused Ultrasound Surgery". Obstetrics & Gynecology. 110 (2, Part 1): 279–87. doi:10.1097/01.AOG.0000275283.39475.f6. PMID 17666601. S2CID 6650678.
- Zhou, Yufeng (2014). "High-Intensity Focused Ultrasound Treatment for Advanced Pancreatic Cancer". Gastroenterology Research and Practice. 2014: 205325. doi:10.1155/2014/205325. ISSN 1687-6121. PMC 4099025. PMID 25053938.
- Jácome-Pita, F; Sánchez-Salas, R; Barret, E; Amaruch, N; Gonzalez-Enguita, C; Cathelineau, X (2014). "Focal therapy in prostate cancer: the current situation". ecancermedicalscience. 8: 435. doi:10.3332/ecancer.2014.435. PMC 4049329. PMID 24944577.
- Diagnostic Ultrasound (4th ed.). Elsevier. 2011. pp. 32–33. ISBN 978-0-323-05397-6.
- "FDA Clears Focused Ultrasound System for Prostate Cancer Treatment". Oncology Times. 37 (22): 37. November 2015. doi:10.1097/01.COT.0000475249.19383.04.
- "510(k) Premarket Notification". www.accessdata.fda.gov.
- "Type 2 classification approval letter" (PDF).
- Chaussy, CG; Thüroff, S (April 2017). "High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review". Journal of Endourology. 31 (S1): S30–S37. doi:10.1089/end.2016.0548. PMID 28355119.
- Hu, Jim C.; Laviana, Aaron; Sedrakyan, Art (28 June 2016). "High-Intensity Focused Ultrasound for Prostate Cancer". JAMA. 315 (24): 2659–60. doi:10.1001/jama.2016.5002. PMID 27367874.
- Lepor, H; Gold, S; Wysock, J (2018). "Focal Ablation of Prostate Cancer". Reviews in Urology. 20 (4): 145–157. doi:10.3909/riu0809 (inactive 2021-01-16). PMC 6375006. PMID 30787673.CS1 maint: DOI inactive as of January 2021 (link)
- Ng, Kelvin K. C.; Poon, Ronnie T. P.; Chan, See Ching; Chok, Kenneth S. H.; Cheung, Tan To; Tung, Helen; Chu, Ferdinand; Tso, Wai Kuen; Yu, Wan Ching; Lo, Chung Mau; Fan, Sheung Tat (May 2011). "High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience". Annals of Surgery. 253 (5): 981–987. doi:10.1097/SLA.0b013e3182128a8b. hdl:10722/135541. ISSN 1528-1140. PMID 21394012. S2CID 25603451.
- Mauri, Giovanni; Nicosia, Luca; Xu, Zhen; Di Pietro, Salvatore; Monfardini, Lorenzo; Bonomo, Guido; Varano, Gianluca Maria; Prada, Francesco; Della Vigna, Paolo; Orsi, Franco (March 2018). "Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer". The British Journal of Radiology. 91 (1083). doi:10.1259/bjr.20170641. ISSN 0007-1285. PMC 5965486. PMID 29168922.
- Qu, Shibin; Worlikar, Tejaswi; Felsted, Amy E.; Ganguly, Anutosh; Beems, Megan V.; Hubbard, Ryan; Pepple, Ashley L.; Kevelin, Alicia A.; Garavaglia, Hannah; Dib, Joe; Toma, Mariam (2020-01-01). "Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy". Journal for ImmunoTherapy of Cancer. 8 (1): e000200. doi:10.1136/jitc-2019-000200. ISSN 2051-1426. PMC 7057529. PMID 31940590.
- Therapeutic Ultrasound. Advances in Experimental Medicine and Biology. New York: Springer. 2016. ISBN 978-3-319-22536-4.
- "Philips Sonalleve receives CE Mark for MR-guided focused ultrasound ablation of metastatic bone cancer" (Press release). Philips Healthcare. April 20, 2011. Archived from the original on October 5, 2013. Retrieved October 4, 2013.
- Wu, F.; Wang, Z.-B.; Zhu, H.; Chen, W.-Z.; Zou, J.-Z.; Bai, J.; Li, K.-Q.; Jin, C.-B.; Xie, F.-L.; Su, H.-B. (2005). "Feasibility of US-guided High-Intensity Focused Ultrasound Treatment in Patients with Advanced Pancreatic Cancer: Initial Experience". Radiology. 236 (3): 1034–40. doi:10.1148/radiol.2362041105. PMID 16055692.
- Madersbacher S, Schatzl G, Djavan B, Stulnig T, Marberger M (2000). "Long-term outcome of transrectal high- intensity focused ultrasound therapy for benign prostatic hyperplasia". Eur Urol. 37 (6): 687–94. doi:10.1159/000020219. PMID 10828669. S2CID 46793601.CS1 maint: multiple names: authors list (link)
- Sommer G, Pauly KB, Holbrook A, Plata J, Daniel B, Bouley D (2013). "Applicators for magnetic resonance-guided ultrasonic ablation of benign prostatic hyperplasia". Invest Radiol. 48 (6): 387–94. doi:10.1097/RLI.0b013e31827fe91e. PMC 4045500. PMID 23462673.CS1 maint: multiple names: authors list (link)
- Salgaonkar VA, Diederich CJ (2015). "Catheter-based ultrasound technology for image-guided thermal therapy: current technology and applications". Int J Hyperth. 31 (2): 203–15. doi:10.3109/02656736.2015.1006269. PMC 4659534. PMID 25799287.
- National Institute for Health and Care Excellence (NICE): Current care pathway (for BPH), August 2018.
- Focused Ultrasound Foundation: Benign Prostatic Hyperplasia (BPH), Website of the PR organization of the industry.
- http://www.accessdata.fda.gov/cdrh_docs/pdf15/DEN150011.pdf%5B%5D%5B%5D
- Zhou, Yu-Feng (2011-01-10). "High intensity focused ultrasound in clinical tumor ablation". World Journal of Clinical Oncology. 2 (1): 8–27. doi:10.5306/wjco.v2.i1.8. ISSN 2218-4333. PMC 3095464. PMID 21603311.
- Therapeutic Ultrasound. Advances in Experimental Medicine and Biology. New York: Springer. 2016. pp. 3–20. ISBN 978-3-319-22536-4.
- Sapareto, Stephen A.; Dewey, William C. (1984). "Thermal dose determination in cancer therapy". International Journal of Radiation Oncology, Biology, Physics. 10 (6): 787–800. doi:10.1016/0360-3016(84)90379-1. PMID 6547421.
- Mouratidis, Petros X. E.; Rivens, Ian; Civale, John; Symonds-Tayler, Richard; Haar, Gail ter (2019-01-01). "'Relationship between thermal dose and cell death for "rapid" ablative and "slow" hyperthermic heating'". International Journal of Hyperthermia. 36 (1): 228–242. doi:10.1080/02656736.2018.1558289. ISSN 0265-6736. PMID 30700171.
- Huisman, Merel; Lam, Mie K; Bartels, Lambertus W; Nijenhuis, Robbert J; Moonen, Chrit T; Knuttel, Floor M; Verkooijen, Helena M; van Vulpen, Marco; van den Bosch, Maurice A (2014). "Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases". Journal of Therapeutic Ultrasound. 2: 16. doi:10.1186/2050-5736-2-16. PMC 4193684. PMID 25309743.
- Köhler, Max O.; Mougenot, Charles; Quesson, Bruno; Enholm, Julia; Le Bail, Brigitte; Laurent, Christophe; Moonen, Chrit T. W.; Ehnholm, Gösta J. (2009). "Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry". Medical Physics. 36 (8): 3521–35. Bibcode:2009MedPh..36.3521K. doi:10.1118/1.3152112. PMID 19746786.
- Monteith, Stephen J.; Kassell, Neal F.; Goren, Oded; Harnof, Sagi (2013). "Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage". Neurosurgical Focus. 34 (5): E14. doi:10.3171/2013.2.FOCUS1313. PMID 23634918.
- Haen, Sebastian P.; Pereira, Philippe L.; Salih, Helmut R.; Rammensee, Hans-Georg; Gouttefangeas, Cécile (2011). "More Than Just Tumor Destruction: Immunomodulation by Thermal Ablation of Cancer". Clinical and Developmental Immunology. 2011: 1–19. doi:10.1155/2011/160250. PMC 3254009. PMID 22242035.
- Wu, Feng (2013). "High intensity focused ultrasound ablation and antitumor immune response". The Journal of the Acoustical Society of America. 134 (2): 1695–701. Bibcode:2013ASAJ..134.1695W. doi:10.1121/1.4812893. PMID 23927210.
- Leighton, T.G. (1997). Ultrasound in food processing. Chapter 9: The principles of cavitation: Thomson Science, London, Blackie Academic and Professional. pp. 151–182.CS1 maint: location (link)
- Levario-Diaz, Victoria; Bhaskar, Pradeep; Carmen Galan, M.; Barnes, Adrian C. (2020-05-22). "Effect of acoustic standing waves on cellular viability and metabolic activity". Scientific Reports. 10 (1): 8493. Bibcode:2020NatSR..10.8493L. doi:10.1038/s41598-020-65241-4. ISSN 2045-2322. PMC 7244593. PMID 32444830.
- Hariharan, P; Myers, M R; Banerjee, R K (21 July 2007). "HIFU procedures at moderate intensities—effect of large blood vessels". Physics in Medicine and Biology. 52 (12): 3493–3513. Bibcode:2007PMB....52.3493H. doi:10.1088/0031-9155/52/12/011. PMID 17664556. S2CID 26124121.
- Izadifar, Zahra; Izadifar, Zohreh; Chapman, Dean; Babyn, Paul (2020-02-07). "An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications". Journal of Clinical Medicine. 9 (2): 460. doi:10.3390/jcm9020460. ISSN 2077-0383. PMC 7073974. PMID 32046072.
- Medel, Ricky; Monteith, Stephen J.; Elias, W. Jeffrey; Eames, Matthew; Snell, John; Sheehan, Jason P.; Wintermark, Max; Jolesz, Ferenc A.; Kassell, Neal F. (2012). "Magnetic Resonance–Guided Focused Ultrasound Surgery". Neurosurgery. 71 (4): 755–763. doi:10.1227/NEU.0b013e3182672ac9. ISSN 0148-396X. PMC 4104674. PMID 22791029.
- Belzberg, Micah; Mahapatra, Smruti; Perdomo-Pantoja, Alexander; Chavez, Francisco; Morrison, Kyle; Xiong, K. Timothy; Gamo, Nao J.; Restaino, Stephen A.; Thakor, Nitish; Yazdi, Youseph; Iyer, Rajiv; Tyler, Betty; Theodore, Nicholas; Luciano, Mark G.; Brem, Henry; Groves, Mari; Cohen, Alan R.; Manbachi, Amir (2020). "Minimally invasive therapeutic ultrasound: Ultrasound-guided ultrasound ablation in neuro-oncology". Ultrasonics. 108 (12): 106210. doi:10.1016/j.ultras.2020.106210. PMID 32619834.
- Cafarelli, A.; Mura, M.; Diodato, A.; Schiappacasse, A.; Santoro, M.; Ciuti, G.; Menciassi, A. (2015). "A computer-assisted robotic platform for Focused Ultrasound Surgery: Assessment of high intensity focused ultrasound delivery". 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2015. pp. 1311–1314. doi:10.1109/EMBC.2015.7318609. ISBN 978-1-4244-9271-8. PMID 26736509. S2CID 4194253.
- Chen, Po-Heng; Hsieh, Kai-Sheng; Huang, Chih-Chung (2017). "An Acoustic Tracking Approach for Medical Ultrasound Image Simulator". Journal of Medical and Biological Engineering. 37 (6): 944–952. doi:10.1007/s40846-017-0258-9. ISSN 1609-0985. PMC 6208925. PMID 30416414.
External links
- Therapeutic Ultrasound at Curlie
- Despite Doubts, Cancer Therapy Draws Patients from The New York Times on 18