Syringol
Syringol is a naturally occurring aromatic organic compound. It is a dimethyl ether of pyrogallol.
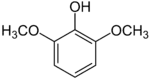 | |
| Names | |
|---|---|
| IUPAC name
1,3-Dimethoxy-2-hydroxybenzene | |
| Other names
Syringol 2,6-Dimethoxyphenol 2-Hydroxy-1,3-dimethoxybenzene Pyrogallol 1,3-dimethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| 1526871 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.856 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H10O3 | |
| Molar mass | 154.16 g/mol |
| Appearance | Gray to light brown solid |
| Density | 1.15857 g/cm3 (60 °C) [2] |
| Melting point | 50 to 57 °C (122 to 135 °F; 323 to 330 K) |
| Boiling point | 262 °C (504 °F; 535 K) [2] |
| Slightly soluble | |
| Vapor pressure | 15.8 Pa (60 °C) [2] |
| Hazards[1] | |
| Flash point | 140 °C (284 °F; 413 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence
Together with guaiacol, syringol and its derivates are characteristic products of pyrolysis of lignin, being derived from the thermal decomposition of the sinapyl alcohol monomer. As such, syringol is an important component of wood smoke.
Uses
Food preparation
In preparation of food by smoking, syringol is the main chemical responsible for the smoky aroma, while guaiacol contributes mainly to taste. Artificial liquid or solid smoke flavorings also contain the chemical, on average composing 13.73% and 13.42% of those products by mass respectively.[3]
Chemical feedstock
Pyrolysis oil, a biofuel derived from woody biomass, can be optimized to yield syringol as a byproduct, potentially replacing demand for petroleum derived phenols.[4] For instance, studies indicate that syringol can serve as a substitute feedstock for phenol formaldehyde resin, a commonly used, water resistant adhesive for plywood.[5]
See also
References
- 2,6-Dimethoxyphenol at Sigma-Aldrich
- Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. doi:10.1007/s10765-019-2570-9.
- Mrak, E. M., Chichester, C. O., & Schweigert (1984). Advances in Food Research, Volume 29. London: Academic Press, Inc. pp. 129–130. ISBN 9780080567488.CS1 maint: uses authors parameter (link)
- Dinesh Mohan; Charles U. Pittman Jr.; Philip H. Steele (2006). "Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review". Energy & Fuels. 20 (3): 863. doi:10.1021/ef0502397.
- Bridgwater, A.V.; Effendi A; Gerhauser H (2008). "Production of renewable phenolic resin by thermochemical conversion of biomass: A review". Renewable and Sustainable Energy Reviews. 12 (8). doi:10.1016/j.rser.2007.04.008.