TMEM169
Transmembrane protein 169 (TMEM169) in humans is encoded by TMEM169 gene.[1] The aliases of TMEM169 include FLJ34263, DKFZp781L2456, and LOC92691.[2] TMEM169 has the highest expression in the brain, particularly the fetal brain.[1] TMEM169 has homologs mammals, reptiles, amphibians, birds, fish, chordates and invertebrates. The most distantly related homolog of TMEM169 is Anopheles albimanus.[3]
| TMEM169 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | TMEM169, transmembrane protein 169 | ||||||||||||||||||||||||
| External IDs | MGI: 2442781 HomoloGene: 16305 GeneCards: TMEM169 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 2: 216.08 – 216.1 Mb | Chr 1: 72.28 – 72.3 Mb | |||||||||||||||||||||||
| PubMed search | [6] | [7] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Gene
Transcripts
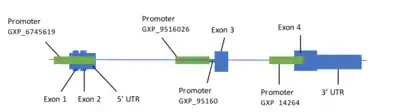
Transcription of TMEM169 gene produces five alternatively spliced variants to generate four variants of the transcript.[1][8] The variants all code for the same protein and have differences within the 5’ UTR region.[1] Variant 1 is the longest transcript (NM_001142310.2)[1] and consists of 3,408 base pairs and four exons.
Protein
All four transcripts encode for the same protein (TMEM169) consisting of 297 amino acids.[1] According to SAPS[9] the molecular weight of protein TMEM169 is 33.6 kdal and it has an overall net negative charge (-5.7%). TMEM169 has an isoelectric point of 4.76.[10]
Domains
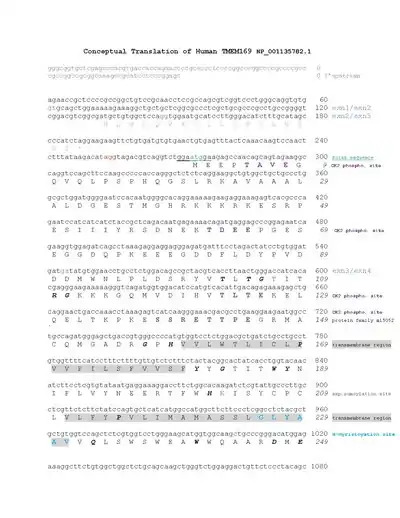
There are two transmembrane domains of Homo sapiens TMEM169 located at amino acids 160-180 and 211-231.[1] The N and C terminus are both located in the cytoplasmic domain.[11] The conceptual translation provides further annotation of Homo sapiens TMEM169 protein.
Secondary Structure
The specific structure of TMEM169 is unknown, although includes both alpha helices and beta sheets.[12]
.pdf.jpg.webp)
Gene level regulation
Promoter
The promoter (GXP_6745619) is 1242 base pairs at coordinates 216080866-216082107.[13] It supports 11 coding transcripts.
| Promoter | Size | Coordinates | Supporting Transcripts |
| GXP_6745619 (+) | 1242 bp | 216080866-216082107 | 11 |
| GXP_9516026 (+) | 1040 bp | 216094326-216095365 | Computational |
| GXP_9516027 (+) | 1040 bp | 216094696-216095735 | Computational |
| GXP_142640 (+) | 1040 bp | 216099253-216100292 | 1 |
Table 2. There are four promoters that result from TMEM169 search on Genomatix.[13]
RNA sequencing
RNA sequencing data revealed TMEM169 is expressed ubiquitously, although it displays high expression in the brain, particularly during fetal development.[1] HPA RNA-seq normal tissues for TMEM169 were obtained from 95 human tissue samples. The HPA RNA-seq demonstrates highest expression of TMEM169 in the brain (RPKM=3.159). Particularly, in the fetal brain (RPKM= 1.583) and cerebellum(RPKM=0.795).[1]
NCBI GEO Profile Microarray
GEO Profile: GDS3113/220527[14]
The highest expression of TMEM169 is found in the fetal brain. TMEM169 tends to have a high expression in the brain relative to other tissues, although not to other genes.
Antibodies
The use of polyclonal TMEM169 antibody (NBP2-69796) of a human melanoma cell line (SK-MEL-30) in the host of rabbit demonstrates TMEM169 localization to nucleoplasm, cytosol and centrosome.[15] Strong positivity in glial cells is revealed through immunohistochemical staining of the human cerebral cortex with antibody HPA074877.[16]
Protein Level Regulation
Post-transalational modifications
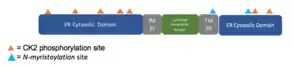
The MyHits[17] Tool revealed two types of conserved predicted post-translational modifications.
| Predicted Modification | Homo Sapiens Amino Acid Location |
|---|---|
| Casein Kinase 2 phosphorylation sites | 5-8, 47-50, 57-60, 62-65, 123-26, 138-141, 142-145, 269-272, 284-287 |
| N-myristoylation site | 226-231, 261-266 |
Homology
TMEM169 is well-conserved across strict orthologs shown by the following multiple sequence alignment created Clustal[18] using BoxShade.[19] The multiple sequence alignment of distant orthologs highlighting the transmembrane domains was also created using Clustal[18] and BoxShade.[19] The red boxes illustrate conserved transmembrane domains across orthologs.

Orthologs
Orthologs are found in over 300 organisms within the kingdom metazoan.[1][8] There are not any orthologs found in fungi or plants. Invertebrates are the most distantly related orthologs, according to BLAST[3] searches. The subset of closely related organisms include mammals, reptiles, birds, and amphibians (E-value 1e-177 to 1e-124). These orthologs range from 78% to 93% sequence similarity to the human protein. Moderately related orthologs to human TMEM169 protein include fish and cephalochordates (similarity= 62%-75%; E-value= 3e-120 to 1e-71). Protein sequences of hemichordates, arthropods, and mollusks are the most distantly related to the human protein sequence (similarity= 56%-64%; E-value= 2e-58 to 1e-44).[3] The most distantly related organism with TMEM169 ortholog is Anopheles albimanus, within the taxonomic group Arthropoda. The size of the gene family for Anopheles albimanus is one and there is only one isoform.[20]

| Scientific Name | Common name | Taxonomic group | Accession Number | Date of Divergence (MYA) | % identity |
|---|---|---|---|---|---|
| Homo sapiens | Human | Mammalia | NP_001135782.1 | 0 | 100 |
| Ictidomys tridecemlineatus | Thirteen-lined ground squirrel | Mammalia | XP_005331895.1 | 90 | 90 |
| Chelonoidis abingdonii | Pinta island tortoise | Reptilla | XP_032620330 | 312 | 69 |
| Pelodiscus sinensis | Chinese softshell turtle | Reptilia | XP_006124795 | 312 | 68 |
| Phaethon lepturus | White tailed tropic bird | Aves | XP_010279821.1 | 312 | 67 |
| Gallus gallus | Chicken | Aves | XP_015145533.1 | 318 | 66 |
| Rhinatrema bivittatum | Two-lined caecilian | Amphibia | XP_029460980.1 | 352 | 66 |
| Nanorana parkeri | High himalaya frog | Amphibia | XP_018409958.1 | 353 | 65 |
| Danio rerio | Zebrafish | Actinopterygii | NP_001313389.1 | 435 | 50 |
| Callorhinchus milii | Australian ghostshark | Chondrichthyes | XP_007908296.1 | 473 | 64 |
| Rhincodon typus | Whale shark | Chondrichthyes | XP_020389226.1 | 473 | 60 |
| Petromyzon marinus | Sea lamprey | Petrymyzontidae | XP_032801904.1 | 615 | 51 |
| Acanthaster planci | Crown of thorns starfish | Echinodermata | XP_022081757.1 | 627 | 40 |
| Brachiostoma floridae | Florida lancelet | Cephalochordata | XP_035657488.1 | 684 | 54 |
| Saccoglossus kowalevskii | Acorn worm | Hemichordata | XP_002740626 | 684 | 45 |
| Drosophila melangogaster | Fruit fly | Arthropoda | NP_650031.1 | 797 | 45 |
| Limulus polyphemus | Atlantic horseshoe crab | Arthropoda | XP_022256315 | 797 | 51 |
| Octopus vulgaris | Octopus | Mollusca | XP_029635540.1 | 797 | 41 |
| Lingula anatina | Lingula anantina | Mollusca | XP_013413871.1 | 797 | 39 |
| Anopheles albimanus | Mosquito strain | Arthropoda | XP_035794912.1 | 797 | 38 |
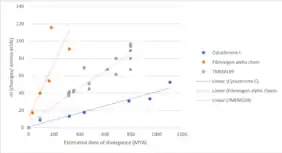
Table 1. Identified orthologs of Homo sapiens TMEM169 protein. The table is organized by increasing DOD (data of divergence from the human lineage) and then by sequence similarity to the human protein (%). The date of divergence was calculated using TimeTree.[21] Seq. is an abbreviation for sequence.
Evolutionary History
TMEM169 gene first appeared in invertebrates, more specifically in arthropods and mollusks. The approximate date of divergence from humans for arthropods and mollusks is 797 million years ago (MYA).[22] Based upon comparison of the evolution for cytochrome C (a quickly evolving protein) and fibrinogen alpha chain (a slowly evolving protein), TMEM169 gene is evolving at a moderate pace.
Interacting Proteins
RhoU (rho-related GTP-binding protein RhoU) is known to interact with TMEM169 according to PSICQUIC.[23] The pull down assay revealed RhoU as a protein interaction with TMEM169.[24]
Clinical Relevance- Disease Association
A single nucleotide polymorphism of TMEM169, defined as rs3821104, is one of two SNP’s that revealed strong association (p<0.1) with Chronic Obstructive Pulmonary Disease (COPD) within the international COPD genetics network.[25] Additionally, gene TMEM169 is one of the four most down-regulated genes in response to oxidative stress; it is downregulated by 0.50 fold in patients with myocardial infarctions.[26] The gene has association to two different birth defects; neural tube defects and preeclampsia. An amino acid change (p.K41I) in TMEM169 is known to cause neural tube defects[27] and hypermethylated loci cg20968678, associated with TMEM169, leads to early onset preeclampsia.[28]
References
- "TMEM169 transmembrane protein 169 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-09-29.
- "AceView: Gene:TMEM169, a comprehensive annotation of human, mouse and worm genes with mRNAs or ESTsAceView". www.ncbi.nlm.nih.gov. Retrieved 2020-09-29.
- "BLAST: Basic Local Alignment Search Tool". blast.ncbi.nlm.nih.gov. Retrieved 2020-10-22.
- GRCh38: Ensembl release 89: ENSG00000163449 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000026188 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "TMEM169 Gene - GeneCards | TM169 Protein | TM169 Antibody". www.genecards.org. Retrieved 2020-09-29.
- "SAPS < Sequence Statistics < EMBL-EBI". www.ebi.ac.uk. Retrieved 2020-12-16.
- "SIB Swiss Institute of Bioinformatics | Expasy". www.expasy.org. Retrieved 2020-12-16.
- "Phyre 2 alignment of Undefined with c5doqC_". www.sbg.bio.ic.ac.uk. Retrieved 2020-12-18.
- "Phyre 2 alignment of Undefined with c5doqC_". www.sbg.bio.ic.ac.uk. Retrieved 2020-12-16.
- "Genomatix Software Suite - Scientific Analysis of genomic data". www.genomatix.de. Retrieved 2020-12-07.
- "49003965 - GEO Profiles - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-12-07.
- "TMEM169 Antibodies". Novus Biologicals. Retrieved 2020-12-07.
- "Tissue expression of TMEM169 - Staining in cerebral cortex - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2020-12-07.
- "Motif Scan". myhits.sib.swiss. Retrieved 2020-12-16.
- "Clustal Omega < Multiple Sequence Alignment < EMBL-EBI". www.ebi.ac.uk. Retrieved 2020-10-29.
- "BoxShade Server". embnet.vital-it.ch. Retrieved 2020-10-29.
- "transmembrane protein 169-like [Anopheles albimanus] - Protein - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-10-22.
- "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2020-10-29.
- "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2020-10-22.
- "PSICQUIC View". www.ebi.ac.uk. Retrieved 2020-12-16.
- Dart AE, Box GM, Court W, Gale ME, Brown JP, Pinder SE, et al. (November 2015). "PAK4 promotes kinase-independent stabilization of RhoU to modulate cell adhesion". The Journal of Cell Biology. 211 (4): 863–79. doi:10.1083/jcb.201501072. PMC 4657161. PMID 26598620.
- Hersh CP, Pillai SG, Zhu G, Lomas DA, Bakke P, Gulsvik A, et al. (September 2010). "Multistudy fine mapping of chromosome 2q identifies XRCC5 as a chronic obstructive pulmonary disease susceptibility gene". American Journal of Respiratory and Critical Care Medicine. 182 (5): 605–13. doi:10.1164/rccm.200910-1586OC. PMC 2937234. PMID 20463177.
- Yue X, Acun A, Zorlutuna P (August 2017). "Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system". Acta Biomaterialia. 58: 337–348. doi:10.1016/j.actbio.2017.06.031. PMC 5563470. PMID 28648749.
- Pappa L, Kals M, Kivistik PA, Metspalu A, Paal A, Nikopensius T (September 2017). "Exome analysis in an Estonian multiplex family with neural tube defects-a case report". Child's Nervous System. 33 (9): 1575–1581. doi:10.1007/s00381-017-3491-1. PMID 28721594.
- Ching T, Ha J, Song MA, Tiirikainen M, Molnar J, Berry MJ, et al. (2015-03-13). "Genome-scale hypomethylation in the cord blood DNAs associated with early onset preeclampsia". Clinical Epigenetics. 7 (1): 21. doi:10.1186/s13148-015-0052-x. PMC 4371797. PMID 25806090.