Tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus.[4] Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydroxybutanedioic acid | |
| Other names
Tartaric acid 2,3-Dihydroxysuccinic acid Threaric acid Racemic acid Uvic acid Paratartaric acid Winestone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.903 |
| E number | E334 (antioxidants, ...) |
| KEGG | |
| MeSH | tartaric+acid |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
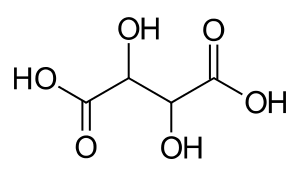
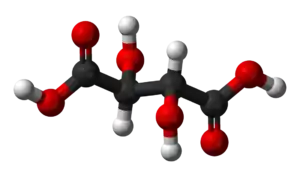
| C4H6O6 (Basic formula) HO2CCH(OH)CH(OH)CO2H (Structural formula) | |
| Molar mass | 150.087 g/mol |
| Appearance | White powder |
| Density | 1.79 g/mL (H2O) |
| Melting point | 171 to 174 °C (340 to 345 °F; 444 to 447 K) (L or D-tartaric; pure) 206 °C (DL, racemic) 165–166 °C (meso-anhydrous) 146–148 °C (meso-hydrous)[2] |
| |
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.89, pKa2= 4.40 meso 25 °C: pKa1= 3.22, pKa2= 4.85 |
| Conjugate base | Bitartrate |
| −67.5·10−6 cm3/mol | |
| Hazards | |
EU classification (DSD) (outdated) |
Irritant(Xi) |
| R-phrases (outdated) | R36 |
| Related compounds | |
Other cations |
Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
Related carboxylic acids |
Butyric acid Succinic acid Dimercaptosuccinic acid Malic acid Maleic acid Fumaric acid |
Related compounds |
2,3-Butanediol Cichoric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tartaric acid is an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.
History
Tartaric acid has been known to winemakers for centuries. However, the chemical process for extraction was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.[5]
Tartaric acid played an important role in the discovery of chemical chirality. This property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light.[6][7] Louis Pasteur continued this research in 1847 by investigating the shapes of sodium ammonium tartrate crystals, which he found to be chiral. By manually sorting the differently shaped crystals, Pasteur was the first to produce a pure sample of levotartaric acid.[8][9][10][11][12]
Stereochemistry

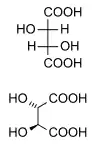
Naturally occurring tartaric acid is chiral, and is a useful raw material in organic chemical synthesis. The naturally occurring form of the acid is dextrotartaric acid or L-(+)-tartaric acid (obsolete name d-tartaric acid). Because it is available naturally, it is slightly cheaper than its enantiomer and the meso isomer. The dextro and levo prefixes are archaic terms.[13] Modern textbooks refer to the natural form as (2R,3R)-tartaric acid (L-(+)-tartaric acid), and its enantiomer as (2S,3S)-tartaric acid (D-(-)-tartaric acid). The meso diastereomer is (2R,3S)-tartaric acid (which is identical with ‘(2S,3R)-tartaric acid’).
Whereas the two chiral stereoisomers rotate plane polarized light in opposite directions, solutions of meso-tartaric acid do not rotate plane-polarized light. The absence of optical activity is due to a mirror plane in the molecule [segmented line in picture below].[14][15]
Tartaric acid in Fehling's solution binds to copper(II) ions, preventing the formation of insoluble hydroxide salts.
| DL-tartaric acid (racemic acid) (when in 1:1 ratio) | mesotartaric acid | |
|---|---|---|
| dextrotartaric acid (L-(+)-tartaric acid) |
levotartaric acid (D-(−)-tartaric acid) | |
 |
 |
 |
| Common name | Tartaric acid | Levotartaric acid | Dextrotartaric acid | Mesotartaric acid | Racemic acid |
|---|---|---|---|---|---|
| Synonyms | (2S,3S)-tartaric acid (S,S)-tartaric acid (−)-tartaric acid l-tartaric acid (obsolete) levotartaric acid D-tartaric acid D-threaric acid ('unnatural isomer')[16] |
(2R,3R)-tartaric acid (R,R)-tartaric acid (+)-tartaric acid d-tartaric acid (obsolete) L-tartaric acid L-threaric acid (‘natural isomer’)[17] |
(2R,3S)-tartaric acid meso-tartaric acid erythraric acid |
rac-(2R,3S)-tartaric acid (2RS,3SR)-tartaric acid (±)-tartaric acid DL-tartaric acid dl-tartaric acid (obsolete) paratartaric acid uvic acid | |
| PubChem | CID 875 from PubChem | CID 439655 from PubChem | CID 444305 from PubChem | CID 78956 from PubChem | CID 5851 from PubChem |
| EINECS number | |||||
| CAS number | 526-83-0 | 147-71-7 | 87-69-4 | 147-73-9 | 133-37-9 |
Production
L-(+)-Tartaric acid
The L-(+)-tartaric acid isomer of tartaric acid is industrially produced in the largest amounts. It is obtained from lees, a solid byproduct of fermentations. The former byproducts mostly consist of potassium bitartrate (KHC4H4O6). This potassium salt is converted to calcium tartrate (CaC4H4O6) upon treatment with milk of lime (Ca(OH)2):[18]
- KO2CCH(OH)CH(OH)CO2H + Ca(OH)2 → Ca(O2CCH(OH)CH(OH)CO2) + KOH + H2O
In practice, higher yields of calcium tartrate are obtained with the addition of calcium chloride. Calcium tartrate is then converted to tartaric acid by treating the salt with aqueous sulfuric acid:
- Ca(O2CCH(OH)CH(OH)CO2) + H2SO4 → HO2CCH(OH)CH(OH)CO2H + CaSO4
Racemic tartaric acid
Racemic tartaric acid (i.e.: a 50:50 mixture of D-(−)-tartaric acid and L-(+)-tartaric acid molecules, racemic acid) can be prepared in a multistep reaction from maleic acid. In the first step, the maleic acid is epoxidized by hydrogen peroxide using potassium tungstate as a catalyst.[18]
- HO2CC2H2CO2H + H2O2 → OC2H2(CO2H) 2
In the next step, the epoxide is hydrolyzed.
- OC2H2(CO2H)2 + H2O → (HOCH)2(CO2H)2
meso-Tartaric acid
meso-Tartaric acid is formed via thermal isomerization. dextro-Tartaric acid is heated in water at 165 °C for about 2 days. meso-Tartaric acid can also be prepared from dibromosuccinic acid using silver hydroxide:[19]
- HO2CCHBrCHBrCO2H + 2 AgOH → HO2CCH(OH)CH(OH)CO2H + 2 AgBr
meso-Tartaric acid can be separated from residual racemic acid by crystallization, the racemate being less soluble.
Reactivity
L-(+)-tartaric acid, can participate in several reactions. As shown the reaction scheme below, dihydroxymaleic acid is produced upon treatment of L-(+)-tartaric acid with hydrogen peroxide in the presence of a ferrous salt.
- HO2CCH(OH)CH(OH)CO2H + H2O2 → HO2CC(OH)C(OH)CO2H + 2 H2O
Dihydroxymaleic acid can then be oxidized to tartronic acid with nitric acid.[20]
Derivatives


Important derivatives of tartaric acid include its salts, cream of tartar (potassium bitartrate), Rochelle salt (potassium sodium tartrate, a mild laxative), and tartar emetic (antimony potassium tartrate).[21][22][23] Diisopropyl tartrate is used as a co-catalyst in asymmetric synthesis.
Tartaric acid is a muscle toxin, which works by inhibiting the production of malic acid, and in high doses causes paralysis and death.[24] The median lethal dose (LD50) is about 7.5 grams/kg for a human, 5.3 grams/kg for rabbits, and 4.4 grams/kg for mice.[25] Given this figure, it would take over 500 g (18 oz) to kill a person weighing 70 kg (150 lb), so it may be safely included in many foods, especially sour-tasting sweets. As a food additive, tartaric acid is used as an antioxidant with E number E334; tartrates are other additives serving as antioxidants or emulsifiers.
When cream of tartar is added to water, a suspension results which serves to clean copper coins very well, as the tartrate solution can dissolve the layer of copper(II) oxide present on the surface of the coin. The resulting copper(II)-tartrate complex is easily soluble in water.
Tartaric acid in wine

Tartaric acid may be most immediately recognizable to wine drinkers as the source of "wine diamonds", the small potassium bitartrate crystals that sometimes form spontaneously on the cork or bottom of the bottle. These "tartrates" are harmless, despite sometimes being mistaken for broken glass, and are prevented in many wines through cold stabilization (which is not always preferred since it can change the wine's profile). The tartrates remaining on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
Tartaric acid plays an important role chemically, lowering the pH of fermenting "must" to a level where many undesirable spoilage bacteria cannot live, and acting as a preservative after fermentation. In the mouth, tartaric acid provides some of the tartness in the wine, although citric and malic acids also play a role.
Tartaric acid in citrus
Results from a study showed that in citrus, fruits produced in organic farming contain higher levels of tartaric acid than fruits produced in conventional agriculture.[26]
In superconductors
Tartaric acid seems to increase the critical temperature in certain superconductors, by supposedly raising the oxidation grade, while the mechanism of this phenomenon is still not precisely known.[27]
Applications
Tartaric acid and its derivatives have a plethora of uses in the field of pharmaceuticals. For example, it has been used in the production of effervescent salts, in combination with citric acid, to improve the taste of oral medications.[20] The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant.
Tartaric acid also has several applications for industrial use. The acid has been observed to chelate metal ions such as calcium and magnesium. Therefore, the acid has served in the farming and metal industries as a chelating agent for complexing micronutrients in soil fertilizer and for cleaning metal surfaces consisting of aluminium, copper, iron, and alloys of these metals, respectively.[18]
References
- Tartaric Acid – Compound Summary, PubChem.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- Dawson, R.M.C. et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- Duarte, A.M.; Caixeirinho, D.; Miguel, M.G.; Sustelo, V.; Nunes, C.; Fernandes, M.M.; Marreiros, A. (2012). "Organic Acids Concentration in Citrus Juice from Conventional Versus Organic Farming". Acta Horticulturae (933): 601–606. doi:10.17660/actahortic.2012.933.78. ISSN 0567-7572.
- Retzius, Anders Jahan (1770) "Försök med vinsten och dess syra" (Experiments with cream of tartar and its acid), Kungliga Vetenskapsakademiens Handlingar (Proceedings of the Royal Academy of Sciences), 31 : 207–213. From p. 209: "§. 6. Dessa försök omtalte jag för Hr. Carl Wilhelm Scheele (en snabb och lårgirug Pharmaciæ Studiosus) … " (§. 6. I mention these experiments on behalf of Mr. Carl Wilhelm Scheele (a quick and studious student of pharmacology) … )
- Biot (1835) "Mémoire sur la polarization circulaire et sur ses applications à la chimie organique" (Memoir on circular polarization and on its applications to organic chemistry), Mémoires de l'Académie des sciences de l'Institut, 2nd series, 13 : 39–175. That tartaric acid (acide tartarique cristallisé) rotates plane-polarized light is shown in Table G following p. 168. (Note: This article was read to the French Royal Academy of Sciences on 1832 November 5.)
- Biot (1838) "Pour discerner les mélanges et les combinaisons chimiques définies ou non définies, qui agissent sur la lumière polarisée; suivies d'applications aux combinaisons de l'acide tartarique avec l'eau, l'alcool et l'esprit de bois" (In order to discern mixtures and chemical combinations, defined or undefined, which act on polarized light; followed by applications to combinations of tartaric acid with water, alcohol [i.e., ethanol], and spirit of wood [i.e., methanol]), Mémoires de l'Académie des sciences de l'Institut, 2nd series, 15 : 93–279.
- L. Pasteur (1848) "Mémoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire" (Memoir on the relationship which can exist between crystalline form and chemical composition, and on the cause of rotary polarization)," Comptes rendus de l'Académie des sciences (Paris), 26 : 535–538.
- L. Pasteur (1848) "Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire" (On the relations that can exist between crystalline form, and chemical composition, and the sense of rotary polarization), Annales de Chimie et de Physique, 3rd series, 24 : 442–459.
- Pasteur, Louis (1850) "Recherches sur les propriétés spécifiques des deux acides qui composent l'acide racémique" (Investigations into the specific properties of the two acids that compose racemic acid), Annales de Chimie et de Physique, 3rd series, 28 (3) : 56–99. See also Plate II. (See also the report of the commission that was appointed to verify Pasteur's findings, pp. 99–117.) [in French]
- George B. Kauffman and Robin D. Myers (1998). "Pasteur's resolution of racemic acid: A sesquicentennial retrospect and a new translation" (PDF). The Chemical Educator. 3 (6): 1–4. doi:10.1007/s00897980257a. S2CID 95862598. Archived from the original (PDF) on 2006-01-17.
- H. D. Flack (2009). "Louis Pasteur's discovery of molecular chirality and spontaneous resolution in 1848, together with a complete review of his crystallographic and chemical work" (PDF). Acta Crystallographica A. 65 (5): 371–389. doi:10.1107/S0108767309024088. PMID 19687573. Archived from the original (PDF) on 2012-09-06.
- J. M. McBride's Yale lecture on history of stereochemistry of tartaric acid, the D/L and R/S systems
- various (2007-07-23). Organic Chemistry. Global Media. p. 65. ISBN 978-81-89940-76-8. Retrieved 2010-06-05.
- "(WO/2008/022994) Use of azabicyclo hexane derivatives".
- "Tartaric Acid_1".
- "Tartaric Acid_2".
- J.-M. Kassaian "Tartaric acid" in Ullmann's Encyclopedia of Industrial Chemistry; VCH: Weinheim, Germany, 2002, 35, 671-678. doi:10.1002/14356007.a26_163
- Augustus Price West. Experimental Organic Chemistry. World Book Company: New York, 1920, 232-237.
- Blair, G. T.; DeFraties, J. J. (2000). "Hydroxy Dicarboxylic Acids". Kirk Othmer Encyclopedia of Chemical Technology. pp. 1–19. doi:10.1002/0471238961.0825041802120109.a01. ISBN 0471238961.
- Zalkin, Allan; Templeton, David H.; Ueki, Tatzuo (1973). "Crystal structure of l-tris(1,10-phenathroline)iron(II) bis(antimony(III) d-tartrate) octahydrate". Inorganic Chemistry. 12 (7): 1641–1646. doi:10.1021/ic50125a033.
- Haq, I; Khan, C (1982). "Hazards of a traditional eye-cosmetic--SURMA". JPMA. The Journal of the Pakistan Medical Association. 32 (1): 7–8. PMID 6804665.
- McCallum, RI (1977). "President's address. Observations upon antimony". Proceedings of the Royal Society of Medicine. 70 (11): 756–63. doi:10.1177/003591577707001103. PMC 1543508. PMID 341167.
- Alfred Swaine Taylor, Edward Hartshorne (1861). Medical jurisprudence. Blanchard and Lea. p. 61.
- Joseph A. Maga, Anthony T. Tu (1995). Food additive toxicology. CRC Press. pp. 137–138. ISBN 0-8247-9245-9.
- Duarte, A.M.; Caixeirinho, D.; Miguel, M.G.; Sustelo, V.; Nunes, C.; Fernandes, M.M.; Marreiros, A. (2012). "Organic Acids Concentration in Citrus Juice from Conventional Versus Organic Farming". Acta Horticulturae (933): 601–606. doi:10.17660/actahortic.2012.933.78. ISSN 0567-7572.
- arXiv, Emerging Technology from the. "Red Wine, Tartaric Acid, and the Secret of Superconductivity". MIT Technology Review. Retrieved 2020-01-09.