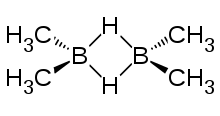
Tetramethyldiborane
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2.[2] Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms.[3] The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.[4][5]
 | |
| Names | |
|---|---|
| IUPAC name
Tetramethyldiborane(6) | |
| Other names
Dimethylborane dimer | |
| Identifiers | |
3D model (JSmol) |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (CH 3) 2BH 2B(CH 3) 2 | |
| Molar mass | 83.777 |
| Appearance | Colorless liquid |
| Odor | Pungent; |
| Melting point | −72.5 °C (−98.5 °F; 200.7 K) |
| Boiling point | 68.6 °C (155.5 °F; 341.8 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| trimethylborane dimethyldiborane diethylborane | |
Related compounds |
Borane tetramethyl aluminium hydride tetramethyl gallium hydride methylalane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formation
Dimethylborane is formed when lithium dimethylborohydride Li(CH3)2BH2 reacts with an acid.[6] The lithium dimethylborohydride can be made from a dimethylborinic ester and lithium monoethoxy aluminium hydride.[6]
Methylboranes are also formed by the reaction of diborane and trimethylborane. This reaction produces four different substitutions of methyl with hydrogen on diborane. Produced is 1-methyldiborane, 1,1-dimethyldborane, 1,1,2-trimethyldiborane and 1,1,2,2-tetramethyldiborane.[3] The latter is maximised when trimethylborane is six times the concentration of diborane.[7]
Other methods to form methyldiboranes include reacting hydrogen with trimethylborane between 80 and 200 °C under pressure, or reacting a metal borohydride with trimethylborane in the presence of hydrogen chloride, aluminium chloride or boron trichloride. If the borohydride is sodium borohydride, then methane is a side product. If the metal is lithium then no methane is produced.[4] dimethylchloroborane and methyldichloroborane are also produced as gaseous products.[4]
Atomic hydrogen converts trimethylborane on a graphene monolayer surface to dimethylborane which dimerises to tetramethyldiborane.[8]
Properties
Tetramethyldiborane has two boron atoms linked by a two hydrogen atom bridge, and each boron is linked to two methyl groups. A Tetramethyldiborane molecule belongs to the D2h point group. Its infrared spectrum shows a strong absorption band at 1602 cm−1 due to bridging hydrogen, a weak band at 1968 cm−1 and lines due to methyl between 900 and 1400 cm−1.[9] In the molecule the boron to hydrogen distance is 1.36 Å, the boron to boron distance is 1.84 Å; the boron to carbon distance is 1.590 Å; the angle of boron-boron to carbon is 120.0°; the boron-carbon-hydrogen angle is 112.0°.[10] The NMR J coupling between two boron-11 nuclei in tetramethyldiborane is 55 Hz.[11]
Tetramethyldiborane melts at -72.5 °C and boils at 68.6 °C.[12] Vapour pressure is approximated by Log P = 7.687-(1643/T).[12] Tetramethyldiborane has a vapour pressure of 48 mm Hg at 0 °C.[7] Heat of vapourisation was measured at 7.3 kcal/mol.[13] The predicted heat of formation for the liquid is ΔH0f=-65 kcal/mol, and for the gas -57 kcal/mol.[13]
A gas chromatograph can be used to determine the amounts of the methyl boranes in a mixture. The order they pass through are diborane, monomethyldiborane, trimethylborane, 1,1-dimethyldiborane, 1,2-dimethyldiborane, trimethyldiborane, and lastly tetramethyldiborane.[14]
The nuclear resonance shift for the bridge hydrogen is 8.90 ppm, compared to 10.49 for diborane.[15]
Reactions
Dimethylborane reacts with alkenes with the highest yield in ether to produce a dimethylalkylborane.[6] The dimethylalkylboranes can then be converted to a tertiary alcohol by oxidative carbonylation. This requires heating to 150° with carbon monoxide under 50 bars of pressure, and then oxidation with hydrogen peroxide.[16]
Methylboranes such as tetramethyldiborane disproportionate in the gas phase to trimethylborane and diborane at room temperature.[3] The time period is on the order of a few hours, and disproportionation is faster the higher the temperature.[4] At 0 °C disproportionation takes about a day.[4] At -78.5 °C methyldiborane disproportionates slowly first to diborane and 1,1-dimethyldiborane.[17] In solution methylborane is more stable against disproportionation than dimethylborane.[6]
- 4(CH3)3B2H3 ⇌ (CH3)4B2H2 + B2H6 K=0.0067.[18]
- 3B2H2Me4 ⇌ 2 B2H3Me3 + 2 BMe3
Dimethylborane is hydrolyzed in water to Dimethylborinic acid (CH3)2BOH.[3]
Dimethyldiborane spontaneously inflames when exposed to air.[17]
Ammonia and tetramethyldiborane combine to form a white solid at -78 °C. The solid decomposes above 10 °C.[19] The structure of the solid is ionic [(CH3)2B(NH3)2]+ [(CH3)2BH2]−.[19][20] A simple adduct BHMe3.N3 is formed from tetramethyldiborane and ammonia in ether. This also forms during the thermal decomposition of the diammoniate.[21]
Acetonitrile reacts slowly with tetramethyldiborane at room temperature to form dimeric ethylideneaminodimethylborane (CH3CH=NB(CH3)2)2. This has a cis and a trans isomer, one melting at 76 °C and another at -5 °C.[22]
Tetramethyldiborane reacts with sodium in liquid ammonia to make a salt with formula Na2HB(CH3)2 called sodium dimethylboryl. The salt is white and stable to 90 °C.[21] With potassium K2HB(CH3)2 potassium dimethylboryl is formed.[23] Calcium metal react with tetramethyldiborane to make CaHB(CH3)2.NH3.[21]
Tetramethyldiborane combines with dimethylphosphine to yield an adduct of dimethylborane.[21]
Tetramethyldiborane reacts with organic borates to form methylboronic esters.
- 2 (CH3)4B2H2 + 4 B(OR)3 ⇌ 6 CH3(OR)2 + (CH3)2B2H4.[24]
Tetramethyldiborane acts as a catalyst to enable the same results from trimethylborane:
- (CH3)3B + 2 B(OR)3 → 3 CH3(OR)2[24]
Related
The tetramethylborate anion (CH3)4B− only has one boron atom.[25]
References
- Baker, Charles J. (2001). The Fire Fighter's Handbook of Hazardous Materials. Jones & Bartlett Learning. p. 352. ISBN 9780962705212.
- Srebnik, Morris; Cole, Thomas E.; Brown, Herbert C. (January 1987). "Methylborane - a remarkable unhindered monoalkylborane which achieves the controlled sequential hydroboration of representative alkenes". Tetrahedron Letters. 28 (33): 3771–3774. doi:10.1016/s0040-4039(00)96380-9.
- Bell, R. P.; Emeléus, H. J. (1948). "The boron hydrides and related compounds". Quarterly Reviews, Chemical Society. 2 (2): 132. doi:10.1039/QR9480200132.
- Long, L. H.; Wallbridge, M. G. H. (1965). "646. The chemistry of boron. Part VI. New preparative methods and decomposition studies relating to methyldiboranes". Journal of the Chemical Society (Resumed): 3513. doi:10.1039/JR9650003513. (subscription required)
- Schlesinger, H. I.; Walker, A. O. (April 1935). "Hydrides of Boron. IV. The Methyl Derivatives of Diborane". Journal of the American Chemical Society. 57 (4): 621–625. doi:10.1021/ja01307a009.
- Brown, Herbert C.; Cole, Thomas E.; Srebnik, Morris; Kim, Kee Won (December 1986). "Hydroboration. 79. Preparation and properties of methylborane and dimethylborane and their characteristics as hydroborating agents. Synthesis of tertiary alcohols containing methyl groups via hydroboration". The Journal of Organic Chemistry. 51 (25): 4925–4930. doi:10.1021/jo00375a031.
- Carpenter, J.H.; Jones, W.J.; Jotham, R.W.; Long, L.H. (June 1970). "The Raman spectra of the methyldiboranes—I 1,1 dimethyldiborane and tetramethyldiborane". Spectrochimica Acta Part A: Molecular Spectroscopy. 26 (6): 1199–1214. Bibcode:1970AcSpA..26.1199C. doi:10.1016/0584-8539(70)80027-7.
- Horn, A; Biener, J; Küppers, J (September 1998). "A dimerization reaction induced by hydrogen atoms: from B(CH3)3 adsorbed on C/Pt(100) to B2H2(CH3)4". Surface Science. 414 (1–2): 290–297. Bibcode:1998SurSc.414..290H. doi:10.1016/S0039-6028(98)00534-2. (subscription required)
- Cowan, R. D. (1949). "The Infra-Red Spectra of Borine Carbonyl and Tetramethyldiborane". The Journal of Chemical Physics. 17 (2): 218. Bibcode:1949JChPh..17..218C. doi:10.1063/1.1747225.
- Carroll, Benjamin L.; Bartell, Lawrence S. (February 1968). "Molecular structure and internal rotation in tetramethyldiborane. Electron diffraction study". Inorganic Chemistry. 7 (2): 219–222. doi:10.1021/ic50060a009.
- Perras, Frédéric A.; Ewing, William C.; Dellermann, Theresa; Böhnke, Julian; Ullrich, Stefan; Schäfer, Thomas; Braunschweig, Holger; Bryce, David L. (2015). "Spying on the boron–boron triple bond using spin–spin coupling measured from 11B solid-state NMR spectroscopy". Chem. Sci. 6 (6): 3378–3382. doi:10.1039/C5SC00644A. PMC 5657093. PMID 29142694.
- Onak, Thomas (1 January 1966). Stone, F. G. A.; West, Robert (eds.). Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. ISBN 9780080580043. Retrieved 14 August 2015.
- Altschuller, Aubrey P. (4 October 1955). "Calculated Heats of Formation and Combustion of Boron Compounds (Boron, Hydrogen, Carbon, Silicon)" (PDF). NACA Research Memorandum. Cleveland, Ohio: National Advisory Committee for Aeronautics. p. 22. Retrieved 14 August 2015.
- Seely, G. R.; Oliver, J. P.; Ritter, D. M. (December 1959). "Gas-Liquid Chromatographic Analysis of Mixtures Containing Methyldiboranes". Analytical Chemistry. 31 (12): 1993–1995. doi:10.1021/ac60156a032.
- Leach, John B.; Ungermann, Charles B.; Onak, Thomas P. (January 1972). "Proton magnetic resonance studies on methyl and chloro substituted diboranes". Journal of Magnetic Resonance. 6 (1): 74–83. Bibcode:1972JMagR...6...74L. doi:10.1016/0022-2364(72)90088-1.
- Srebnik, Morris; Cole, Thomas E.; Brown, Herbert C. (August 1990). "Hydroboration. 87. Controlled and sequential hydroboration of simple representative alkenes with methylborane in tetrahydrofuran. An examination of the directive effects in the first and second stages of hydroboration". The Journal of Organic Chemistry. 55 (17): 5051–5058. doi:10.1021/jo00304a017.
- Bunting, Roger K. (22 Sep 2009). "55 1-Methyldiborane". In Duward F. Shriver (ed.). Inorganic Syntheses, Volume 19. John Wiley and Sons. pp. 237–238. ISBN 978-0471045427.
- Onak, Thomas (1 January 1966). "Carboranes and Organo-Substituted Boron Hydrides". In Stone, F. G. A.; West, Robert (eds.). Advances in Organometallic Chemistry. New York, London: Academic Press. p. 284. ISBN 9780080580043. Retrieved 19 August 2015.
- Moews, P. C.; Parry, R. W. (September 1966). "The Reactions between Tetramethyldiborane and Ammonia. The Dimethyldiammineboron(III) Cation, the Dihydridodimethylborate Anion, and Ammonia Dimethylborane". Inorganic Chemistry. 5 (9): 1552–1556. doi:10.1021/ic50043a018.
- Zhao, Qianyi; Li, Jiaxuan; Hamilton, Ewan J.M.; Chen, Xuenian (May 2015). "The continuing story of the diammoniate of diborane". Journal of Organometallic Chemistry. 798: 24–29. doi:10.1016/j.jorganchem.2015.05.027.
- Onak, Thomas (2 December 2012). Organoborane Chemistry. Elsevier. pp. 183–184. ISBN 9780323153614. Retrieved 12 August 2015.
- Lloyd, J. E.; Wade, K. (1964). "325. Reactions between dialkylboranes and methyl cyanide. Ethylideneaminodimethylborane and diethylethylideneaminoborane". Journal of the Chemical Society (Resumed): 1649–1654. doi:10.1039/JR9640001649. Retrieved 24 July 2015.
- Adams, Roy M. (September 1959). "Organoboron Compounds" (PDF). Metal-Organic Compounds. Advances in Chemistry. 23. p. 92. doi:10.1021/ba-1959-0023.ch010. ISBN 0-8412-0024-6. Retrieved 17 August 2015.
- Mikhailov, B. M. (April 1962). "The Chemistry Of Diborane". Russian Chemical Reviews. 31 (4): 219. Bibcode:1962RuCRv..31..207M. doi:10.1070/RC1962v031n04ABEH001281.
- "tetramethylborate". ChemSpider. Retrieved 19 August 2015.
