Aluminium chloride
Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
aluminium chloride | |
| Other names
aluminium(III) chloride aluminum trichloride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.371 |
| EC Number |
|
| 1876 | |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| AlCl3 | |
| Molar mass | 133.341 g/mol (anhydrous) 241.432 g/mol (hexahydrate)[1] |
| Appearance | white or pale yellow solid, hygroscopic |
| Density | 2.48 g/cm3 (anhydrous) 2.398 g/cm3 (hexahydrate)[1] |
| Melting point | 180 °C (356 °F; 453 K) (anhydrous, sublimes)[1] 100 °C (212 °F; 373 K) (hexahydrate, dec.)[1] |
| 439 g/l (0 °C) 449 g/l (10 °C) 458 g/l (20 °C) 466 g/l (30 °C) 473 g/l (40 °C) 481 g/l (60 °C) 486 g/l (80 °C) 490 g/l (100 °C) | |
| Solubility | soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride slightly soluble in benzene |
| Vapor pressure | 133.3 Pa (99 °C) 13.3 kPa (151 °C)[2] |
| Viscosity | 0.35 cP (197 °C) 0.26 cP (237 °C)[2] |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12[3] | |
Lattice volume (V) |
0.52996 nm3 |
Formula units (Z) |
6 |
| Octahedral (solid) Tetrahedral (liquid) | |
| Trigonal planar (monomeric vapour) | |
| Thermochemistry | |
Heat capacity (C) |
91.1 J/mol·K[4] |
Std molar entropy (S |
109.3 J/mol·K[4] |
Std enthalpy of formation (ΔfH⦵298) |
−704.2 kJ/mol[4] |
Gibbs free energy (ΔfG˚) |
-628.8 kJ/mol[4] |
| Pharmacology | |
| D10AX01 (WHO) | |
| Hazards | |
| Safety data sheet | See: data page |
| GHS pictograms |  [5] [5] |
| GHS Signal word | Danger |
| H314[5] | |
| P280, P310, P305+351+338[5] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
anhydrous: 380 mg/kg, rat (oral) hexahydrate: 3311 mg/kg, rat (oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[6] |
REL (Recommended) |
2 mg/m3[6] |
IDLH (Immediate danger) |
N.D.[6] |
| Related compounds | |
Other anions |
Aluminium fluoride Aluminium bromide Aluminium iodide |
Other cations |
Boron trichloride Gallium trichloride Indium(III) chloride Magnesium chloride |
Related Lewis acids |
Iron(III) chloride Boron trifluoride |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry.[7] The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Uses
Alkylation and acylation of arenes
AlCl3 is a common Lewis-acid catalyst for Friedel–Crafts reactions, both acylations and alkylations.[8] Important products are detergents and ethylbenzene. These types of reactions are the major use for aluminium chloride, for example, in the preparation of anthraquinone (used in the dyestuffs industry) from benzene and phosgene.[9] In the general Friedel–Crafts reaction, an acyl chloride or alkyl halide reacts with an aromatic system as shown:[8]
The alkylation reaction is more widely used than the acylation reaction, although its practice is more technically demanding. For both reactions, the aluminium chloride, as well as other materials and the equipment, should be dry, although a trace of moisture is necessary for the reaction to proceed.[10] Detailed procedures are available for alkylation[11] and acylation[12][13] of arenes.
A general problem with the Friedel–Crafts reaction is that the aluminium chloride catalyst sometimes is required in full stoichiometric quantities, because it complexes strongly with the products. This complication sometimes generates a large amount of corrosive waste. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites.[7]
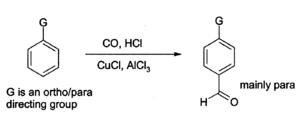
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gattermann-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co-catalyst.[14]
Other applications in organic and organometallic synthesis
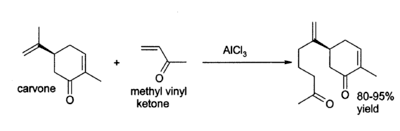
Aluminium chloride finds a wide variety of other applications in organic chemistry.[15] For example, it can catalyse the "ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:[16]
It is used to induce a variety of hydrocarbon couplings and rearrangements.[17][18]
Aluminium chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis. Dichlorophenylphosphine is prepared by reaction of benzene and phosphorus trichloride catalyzed by aluminium chloride.[19]
Structure
Anhydrous
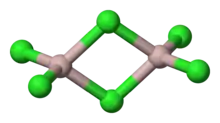
AlCl3 adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 is a sheet-like layered cubic close packed layers. In this framework, the Al centres exhibit octahedral coordination geometry.[20] When aluminium trichloride is in its melted state, it exists as the dimer Al2Cl6, with tetracoordinate aluminium. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) versus solid aluminium trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapour phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3, which is structurally analogous to BF3. The melt conducts electricity poorly,[9] unlike more-ionic halides such as sodium chloride.
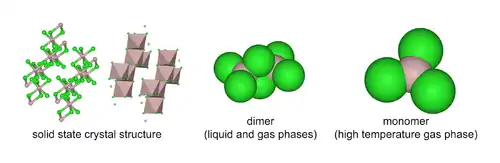
Aluminium chloride monomer belongs to the point group D3h in its monomeric form and D2h in its dimeric form.
Hexahydrate
The hexahydrate consists of octahedral [Al(H2O)6]3+ centers and chloride counterions. Hydrogen bonds link the cation and anions.[21] The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminum ion surrounded by six water ligand molecules. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions.
Reactions
Anhydrous aluminium chloride is a powerful Lewis acid, capable of forming Lewis acid-base adducts with even weak Lewis bases such as benzophenone and mesitylene.[8] It forms tetrachloroaluminate (AlCl4−) in the presence of chloride ions.
Aluminium chloride reacts with calcium and magnesium hydrides in tetrahydrofuran forming tetrahydroaluminates.
Reactions with water
Anhydrous aluminium chloride is hygroscopic, having a very pronounced affinity for water. It fumes in moist air and hisses when mixed with liquid water as the Cl− ligands are displaced with H2O molecules to form the hexahydrate [Al(H2O)6]Cl3 . The anhydrous phase cannot be regained on heating the hexahydrate. Instead HCl is lost leaving aluminium hydroxide or alumina (aluminium oxide):
- Al(H2O)6Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Like metal aquo complexes, aqueous AlCl3 is acidic owing to the ionization of the aquo ligands:
- [Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+
Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions, giving a gelatinous precipitate of aluminium hydroxide upon reaction with dilute sodium hydroxide:
- AlCl3 + 3 NaOH → [Al(OH)3] + 3 NaCl
Synthesis
Aluminium chloride is manufactured on a large scale by the exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures between 650 to 750 °C (1,202 to 1,382 °F).[9]
- 2 Al + 3 Cl2 → 2 AlCl3
- 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminum chloride may be formed via a single displacement reaction between copper chloride and aluminum metal.
- 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.[7]
Hydrated aluminium trichloride is prepared by dissolving aluminium oxides in hydrochloric acid. Metallic aluminum also readily dissolves in hydrochloric acid ─ releasing hydrogen gas and generating considerable heat. Heating this solid does not produce anhydrous aluminium trichloride, the hexahydrate decomposes to aluminium hydroxide when heated:
- Al(H2O)6Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.[9]
Natural occurrence
Anhydrous compound is now unknown among the minerals. The hexahydrate, however, is known as the rare mineral chloraluminite.[22] A more complex, basic and hydrated mineral is cadwaladerite.[23][22]
Safety
Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required. It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact.[24]
See also
References
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.45. ISBN 1439855110.
- Aluminum chloride Archived 2014-05-05 at the Wayback Machine. Chemister.ru (2007-03-19). Retrieved on 2017-03-17.
- Ketelaar, J. A. A. (1935). "Die Kristallstruktur der Aluminiumhalogenide II". Zeitschrift für Kristallographie – Crystalline Materials. 90 (1–6). doi:10.1524/zkri.1935.90.1.237. S2CID 100796636.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.5. ISBN 1439855110.
- Sigma-Aldrich Co., Aluminum chloride. Retrieved on 2014-05-05.
- NIOSH Pocket Guide to Chemical Hazards. "#0024". National Institute for Occupational Safety and Health (NIOSH).
- Helmboldt, Otto; Keith Hudson, L.; Misra, Chanakya; Wefers, Karl; Heck, Wolfgang; Stark, Hans; Danner, Max; Rösch, Norbert (2007). "Aluminum Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_527.pub2.
- Olah, G. A., ed. (1963). Friedel-Crafts and Related Reactions. 1. New York City: Interscience.
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- Nenitzescu, Costin D.; Cantuniari, Ion P. (1933). "Durch Aluminiumchlorid Katalysierte Reaktion, VI. Mitteil.: Die Umlagerung des Cyclohexans in Metyl-cyclopentan". Berichte der deutschen chemischen Gesellschaft (A and B Series). 66 (8): 1097–1100. doi:10.1002/cber.19330660817. ISSN 1099-0682.
- Jonathan T. Reeves1, Zhulin Tan, Daniel R. Fandrick, Jinhua J. Song, Nathan K. Yee, Chris H. Senanayake (2012). "Synthesis of Trifluoromethyl Ketones from Carboxylic Acids: 4-(3,4-Dibromophenyl)-1,1,1-trifluoro-4-methylpentan-2-one". Organic Syntheses. 89: 210. doi:10.15227/orgsyn.089.0210.CS1 maint: multiple names: authors list (link)
- Kamil Paruch; Libor Vyklicky; Thomas J. Katz (2003). "Preparation of 9,10-Dimethoxyphenanthrene and 3,6-Diacetyl-9,10-Dimethoxyphenanthrene". Organic Syntheses. 80: 227. doi:10.15227/orgsyn.080.0227.
- Alexander J. Seed; Vaishali Sonpatki; Mark R. Herbert (2002). "3-(4-Bromobenzoyl)propanoic Acid". Organic Syntheses. 79: 204. doi:10.15227/orgsyn.079.0204.
- Wade, L. G. (2003) Organic Chemistry, 5th edition, Prentice Hall, Upper Saddle River, New Jersey, United States. ISBN 013033832X.
- Galatsis, P. (1999) Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents, H. J. Reich, J. H. Rigby (eds.) Wiley, New York City. pp. 12–15. ISBN 978-0-471-97925-8.
- Snider, B. B. (1980). "Lewis-acid catalyzed ene reactions". Acc. Chem. Res. 13 (11): 426. doi:10.1021/ar50155a007.
- Reuben D. Rieke; Stephen E. Bales; Phillip M. Hudnall; Timothy P. Burns; Graham S. Poindexter (1979). "Highly Reactive Magnesium for the Preparation of Grignard Reagents: 1-Norbornanecarboxylic Acid". Organic Syntheses. 59: 85. doi:10.15227/orgsyn.059.0085.
- Sami A. Shama; Carl C. Wamser (1983). "Hexamethyl Dewar Benzene". Organic Syntheses. 61: 62. doi:10.15227/orgsyn.061.0062.
- B. Buchner; L. B. Lockhart Jr. (1951). "Phenyldichlorophosphine". Organic Syntheses. 31: 88. doi:10.15227/orgsyn.031.0088.
- In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom. ISBN 0198553706.
- Andress, K.R.; Carpenter, C. (1934). "Kristallhydrate II. Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat". Zeitschrift für Kristallographie – Crystalline Materials. 87. doi:10.1524/zkri.1934.87.1.446.
- https://www.ima-mineralogy.org/Minlist.htm
- https://www.mindat.org/min-845.html
- Aluminum Chloride. solvaychemicals.us
External links
| Wikimedia Commons has media related to Aluminium chloride. |
- International Chemical Safety Card 1125
- Index of Organic Synthesis procedures that utilize AlCl3
- The period 3 chlorides
- MSDS
- Government of Canada Fact Sheets and Frequently Asked Questions: Aluminum Salts