Thexylborane
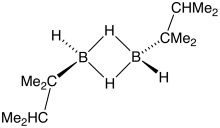
Thexylborane is a borane with the formula [Me2CHCMe2BH2]2 (Me = methyl). A colorless liquid, it is a rare, easily accessed monoalkylborane. It is produced by the hydroboration of tetramethylethylene:[1]
- B2H6 + 2 Me2C=CMe2 → [Me2CHCMe2BH2]2
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| |
| |
| Properties | |
| C12H30B2 | |
| Molar mass | 195.99 g·mol−1 |
| Appearance | colorless liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
Thexylborane is generated in situ. In solution, it isomerizes over the course several days to the 2,3-dimethyl-1-butyl derivative, shown as the monomer here:
- Me2CHCMe2BH2 → Me2CHCH(Me)CH2BH2
Thexylborane allows the atom-economical synthesis of ketones from a pair of alkenes:
- Me2CHCMe2BH2 + 2 RCH=CH2 → Me2CHCH(Me)CH2B(CH2CH2R)2
- Me2CHCH(Me)CH2B(CH2CH2R)2 + CO + H2O → OC(CH2CH2R)2 + ...
The main point is that the thexyl group does not migrate.[1]
References
- Negishi, Ei-Ichi; Brown, Herbert C. (1974). "Thexylborane-A Highly Versatile Reagent for Organic Synthesis via Hydroboration". Synthesis. 1974 (2): 77–89. doi:10.1055/s-1974-23248.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.