Three-finger protein
Three-finger proteins or three-finger protein domains (3FP or TFPD) are a protein superfamily consisting of small, roughly 60-80 amino acid residue protein domains with a common tertiary structure: three beta strand loops extended from a hydrophobic core stabilized by disulfide bonds. The family is named for the outstretched "fingers" of the three loops. Members of the family have no enzymatic activity, but are capable of forming protein-protein interactions with high specificity and affinity. The founding members of the family, also the best characterized by structure, are the three-finger toxins found in snake venom, which have a variety of pharmacological effects, most typically by disruption of cholinergic signaling. The family is also represented in non-toxic proteins, which have a wide taxonomic distribution; 3FP domains occur in the extracellular domains of some cell-surface receptors as well as in GPI-anchored and secreted globular proteins, usually involved in signaling.[2][3][4][5]
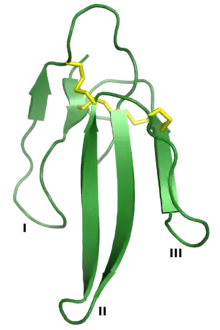
| Three-finger protein | |
|---|---|
| Identifiers | |
| Symbol | ? |
| CATH | 1qkd |
| SCOP2 | 1qkd / SCOPe / SUPFAM |
Three-finger toxins
The founding members of the 3FP family are the three-finger toxins (3FTx) often found in snake venom. 3FTx proteins are widely distributed in venomous snake families, but are particularly enriched in the family Elapidae, in which the relative proportion of 3FTx to other venom toxins can reach 95%.[4][6] Many 3FTx proteins are neurotoxins, though the mechanism of toxicity varies significantly even among proteins of relatively high sequence identity; common protein targets include those involved in cholinergic signaling, such as the nicotinic acetylcholine receptors, muscarinic acetylcholine receptors, and acetylcholinesterase. Another large subfamily of 3FTx proteins is the cardiotoxins (also known as cytotoxins or cytolysins); this group is directly cytotoxic most likely due to interactions with phospholipids and possibly other components of the cell membrane.[2]
Ly6/uPAR family
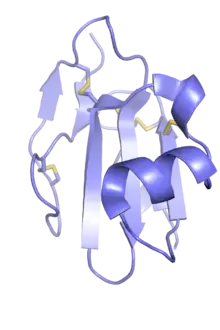
The Ly6/uPAR family broadly describes a gene family containing three-finger protein domains that are not toxic and not venom components; these are often known as LU domains and can be found in the extracellular domains of cell-surface receptors and in either GPI-anchored or secreted globular proteins.[4][8] The family is named for two representative groups of members, the small globular protein lymphocyte antigen 6 (LY6) family and the urokinase plasminogen activator receptor (uPAR).[9] Other receptors with LU domains include members of the transforming growth factor beta receptor (TGF-beta) superfamily, such as the activin type 2 receptor;[10] and bone morphogenetic protein receptor, type IA.[11] Other LU domain proteins are small globular proteins such as CD59 antigen, LYNX1, SLURP1, and SLURP2.[4][12]
Many LU domain containing proteins are involved in cholinergic signaling and bind acetylcholine receptors, notably linking their function to a common mechanism of 3FTx toxicity.[4][8][13] Members of the Ly6/uPAR family are believed to be the evolutionary ancestors of 3FTx toxins.[14] Other LU proteins, such as the CD59 antigen, have well-studied functions in regulation of the immune system.[13]
Gene structure
Snake three-finger toxins and the Ly6/uPAR family members share a common gene structure, typically consisting of two introns and three exons. The sequence of the first exon is generally well conserved compared to the other two.[4] The third exon contains the major differentiating features between the two groups, as this is where the C-terminal GPI-anchor peptide common among the Ly6/uPAR globular proteins is encoded.[4][13]
Evolution and taxonomic distribution
Proteins of the general three-finger fold are widely distributed among metazoans.[4] A 2008 bioinformatics study identified about 45 examples of such proteins, containing up to three three-finger domains, represented in the human genome.[12] A more recent profile of the Ly6/uPAR gene family identified 35 human and at least 61 mouse family members in the organisms' respective genomes.[8]
The three-finger protein family is thought to have expanded through gene duplication in the snake lineage.[14][15] 3FTx toxins are considered restricted to the Caenophidia, the taxon containing all venomous snakes; however at least one homolog has been identified in the Burmese python, a closely related subgroup.[16] Traditionally, 3FTx genes have been thought to have evolved by repeated events of duplication followed by neofunctionalization and recruitment to gene expression patterns restricted to venom glands.[14][15] However, it has been argued that this process should be extremely rare and that subfunctionalization better explains the observed distribution.[17] More recently, non-toxic 3FP proteins have been found to be widely expressed in many different tissues in snakes, prompting the alternative hypothesis that proteins of restricted expression in saliva were selectively recruited for toxic functionality.[16]
References
- Nastopoulos V, Kanellopoulos PN, Tsernoglou D (September 1998). "Structure of dimeric and monomeric erabutoxin a refined at 1.5 A resolution" (PDF). Acta Crystallographica. Section D, Biological Crystallography. 54 (Pt 5): 964–74. doi:10.1107/S0907444998005125. PMID 9757111.
- Kini RM, Doley R (November 2010). "Structure, function and evolution of three-finger toxins: mini proteins with multiple targets". Toxicon. 56 (6): 855–67. doi:10.1016/j.toxicon.2010.07.010. PMID 20670641.
- Hegde RP, Rajagopalan N, Doley R, Kini M (2010). "Snake venom three-finger toxins". In Mackessy SP (ed.). Handbook of venoms and toxins of reptiles. Boca Raton: CRC Press. pp. 287–302. ISBN 9781420008661.
- Kessler P, Marchot P, Silva M, Servent D (August 2017). "The three-finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions". Journal of Neurochemistry. 142 Suppl 2: 7–18. doi:10.1111/jnc.13975. PMID 28326549.
- Utkin Y, Sunagar K, Jackson TN, Reeks T, Fry BG (2015). "Chapter 8: Three-finger toxins". In Fry B (ed.). Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford University Press. pp. 218–227. ISBN 9780199309405.
- Sanz L, Pla D, Pérez A, Rodríguez Y, Zavaleta A, Salas M, Lomonte B, Calvete JJ (June 2016). "Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus tschudii tschudii, Supports the 3FTx/PLA₂ Dichotomy across Micrurus Venoms". Toxins. 8 (6): 178. doi:10.3390/toxins8060178. PMC 4926144. PMID 27338473.
- Leath KJ, Johnson S, Roversi P, Hughes TR, Smith RA, Mackenzie L, Morgan BP, Lea SM (August 2007). "High-resolution structures of bacterially expressed soluble human CD59". Acta Crystallographica. Section F, Structural Biology and Crystallization Communications. 63 (Pt 8): 648–52. doi:10.1107/S1744309107033477. PMC 2335151. PMID 17671359.
- Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S, Swamynathan SK (April 2016). "Organization, evolution and functions of the human and mouse Ly6/uPAR family genes". Human Genomics. 10: 10. doi:10.1186/s40246-016-0074-2. PMC 4839075. PMID 27098205.
- Ploug M, Ellis V (August 1994). "Structure-function relationships in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom alpha-neurotoxins". FEBS Letters. 349 (2): 163–8. doi:10.1016/0014-5793(94)00674-1. PMID 8050560. S2CID 86302713.
- Greenwald J, Fischer WH, Vale WW, Choe S (January 1999). "Three-finger toxin fold for the extracellular ligand-binding domain of the type II activin receptor serine kinase". Nature Structural Biology. 6 (1): 18–22. doi:10.1038/4887. PMID 9886286. S2CID 26301441.
- Kirsch T, Sebald W, Dreyer MK (June 2000). "Crystal structure of the BMP-2-BRIA ectodomain complex". Nature Structural Biology. 7 (6): 492–6. doi:10.1038/75903. PMID 10881198. S2CID 19403233.
- Galat A (November 2008). "The three-fingered protein domain of the human genome". Cellular and Molecular Life Sciences. 65 (21): 3481–93. doi:10.1007/s00018-008-8473-8. PMID 18821057. S2CID 19931506.
- Tsetlin VI (February 2015). "Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators". Trends in Pharmacological Sciences. 36 (2): 109–23. doi:10.1016/j.tips.2014.11.003. PMID 25528970.
- Fry BG (March 2005). "From genome to "venome": molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins". Genome Research. 15 (3): 403–20. doi:10.1101/gr.3228405. PMC 551567. PMID 15741511.
- Fry BG, Casewell NR, Wüster W, Vidal N, Young B, Jackson TN (September 2012). "The structural and functional diversification of the Toxicofera reptile venom system". Toxicon. Advancing in Basic and Translational Venomics. 60 (4): 434–48. doi:10.1016/j.toxicon.2012.02.013. PMID 22446061.
- Reyes-Velasco J, Card DC, Andrew AL, Shaney KJ, Adams RH, Schield DR, Casewell NR, Mackessy SP, Castoe TA (January 2015). "Expression of venom gene homologs in diverse python tissues suggests a new model for the evolution of snake venom". Molecular Biology and Evolution. 32 (1): 173–83. doi:10.1093/molbev/msu294. PMID 25338510.
- Hargreaves AD, Swain MT, Hegarty MJ, Logan DW, Mulley JF (August 2014). "Restriction and recruitment-gene duplication and the origin and evolution of snake venom toxins". Genome Biology and Evolution. 6 (8): 2088–95. doi:10.1093/gbe/evu166. PMC 4231632. PMID 25079342.
External links
- SCOP: SSF57302
- CATH: 2.10.60.10