Tradipitant
Tradipitant (VLY-686 or LY686017) is an experimental drug that is a neurokinin 1 antagonist. It works by blocking substance P, a small signaling molecule. Originally, this compound was owned by Eli Lilly and named LY686017. VLY-686 was purchased by Vanda Pharmaceuticals from Eli Lilly and Company in 2012.[1] Vanda Pharmaceuticals is a U.S. pharmaceutical company that as of November 2015 only has three drugs in their product pipeline: tasimelteon, VLY-686, and iloperidone.[2]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
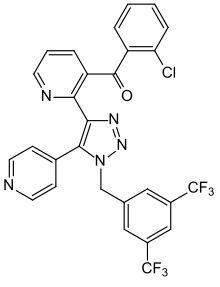
| Formula | C28H16ClF6N5O |
| Molar mass | 587.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Indications
Pruritus
It is being investigated by Vanda Pharmaceuticals for chronic pruritus (itchiness) in atopic dermatitis. In March 2015, Vanda announced positive results from a Phase II proof of concept study.[3] A proof of concept study is done in early stage clinical trials after there have been promising preclinical results. It provides preliminary evidence that the drug is active in humans and has some efficacy.[4]
Alcoholism
VLY-686 reduced alcohol craving in recently detoxified alcoholic patients as measured by the Alcohol Urge Questionnaire.[5] In a placebo controlled clinical trial of recently detoxified alcoholic patients, VLY-686 significantly reduced alcohol craving as measured by the Alcohol Urge Questionnaire. It also reduced the cortisol increase seen after a stress test compared to placebo. The dose given was 50 mg per day.
Social anxiety disorder
In a 12-week randomized trial of LY68017 in 189 patients with social anxiety disorder, 50 mg of LY68017 did not provide any statistically significant improvement over placebo.[6]
Gastroparesis
Tradipitant underwent phase II clinical trials assessing its utility in the treatment of gastroparesis the results of which were announced on December 3rd, 2018. [7] In a study performed over 4 weeks with a patient population of 141 patients, Tradipitant met its primary endpoint of decreased nausea scores as recorded in daily patient diaries. It also showed a statistically significant improvement in nausea-free days, and key gastroparesis scales.
References
- "Company Overview of Eli Lilly & Co., Worldwide License to Develop and Commercialize VLY-686". Bloomberg Business. Retrieved 16 November 2015.
- "Product Pipeline". Vanda Pharmaceuticals Inc. Archived from the original on 27 March 2016.
- "Vanda Pharmaceuticals Announces Tradipitant Phase II Proof of Concept Study Results for Chronic Pruritus in Atopic Dermatitis". PR Newswire. Retrieved 16 November 2015.
- Schmidt B (January 2006). "Proof of Principle studies". Epilepsy Research. 68 (1): 48–52. doi:10.1016/j.eplepsyres.2005.09.019. PMID 16377153. S2CID 7277700.
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. (March 2008). "Neurokinin 1 receptor antagonism as a possible therapy for alcoholism". Science. 319 (5869): 1536–9. Bibcode:2008Sci...319.1536G. doi:10.1126/science.1153813. PMID 18276852.
- Tauscher J, Kielbasa W, Iyengar S, Vandenhende F, Peng X, Mozley D, et al. (February 2010). "Development of the 2nd generation neurokinin-1 receptor antagonist LY686017 for social anxiety disorder". European Neuropsychopharmacology. 20 (2): 80–7. doi:10.1016/j.euroneuro.2009.10.005. PMID 20018493. S2CID 22073069.
- "Vanda Announces Positive Phase II Study Results for Tradipitant in Patients with Gastroparesis". PR Newswire. Retrieved 29 April 2019.
