trans-Dichlorodiammineplatinum(II)
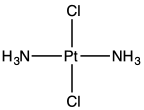
trans-Dichlorodiammineplatinum(II) is the trans isomer of the coordination complex with the formula trans-PtCl2(NH3)2, sometimes called transplatin.[1] It is a yellow solid with low solubility in water but good solubility in DMF. The existence of two isomers of PtCl2(NH3)2 led Alfred Werner to propose square planar molecular geometry.[2] It belongs to the molecular symmetry point group D2h.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Reiset's second chloride, transplatin | |||
| Identifiers | |||
3D model (JSmol) |
| ||
| ECHA InfoCard | 100.035.422 | ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| Cl2H6N2Pt | |||
| Molar mass | 300.05 g·mol−1 | ||
| Appearance | yellow solid | ||
| low | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and reactions
The complex is prepared by treating [Pt(NH3)4]Cl2 with hydrochloric acid.[2]
Many of the reactions of this complex can be explained by the trans effect. It slowly hydrolyzes in aqueous solution to give the mixed aquo complex trans-[PtCl(H2O)(NH3)2]Cl. Similarly it reacts with thiourea (tu) to give colorless trans-[Pt(tu)2(NH3)2]Cl2. In contrast, the cis isomer gives [Pt(tu)4]Cl2. Oxidative addition of chlorine gives trans-PtCl4(NH3)2.
Medicinal chemistry
trans-Dichlorodiammineplatinum(II) has had far less impact on medicinal chemistry compared to its cis isomer, cisplatin, which is a major anticancer drug. Nonetheless, replacement of the ammonia with other ligands has led to highly active drugs that have attracted much attention.[3]
References
- Nakata, B; Yamagata, S; Kanehara, I; Shirasaka, T; Hirakawa, K (25 June 2006). "Transplatin, a cisplatin trans-isomer, may enhance the anticancer effect of 5-fluorouracil". Journal of Experimental & Clinical Cancer Research: CR. 25 (2): 195–200. PMID 16918130.
- Kauffman, George B; Cowan, Dwaine O; Slusarczuk, George; Kirschner, Stanley (1963). "cis- and trans-Dichlorodiammineplatinum(II)". Inorg. Synth. Inorganic Syntheses. 7: 239–245. doi:10.1002/9780470132388.ch63. ISBN 9780470132388.
- Aris, S. M; Farrell, N. P (2009). "Towards Antitumor Active trans-Platinum Compounds". European Journal of Inorganic Chemistry. 2009 (10): 1293–1302. doi:10.1002/ejic.200801118. PMC 2821104. PMID 20161688.