Trinitroethylorthoformate
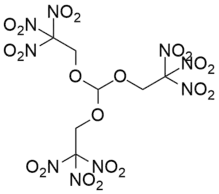
Trinitroethylorthoformate also known as TNEOF is an explosive with excellent chemical stability. It does not have hygroscopicity, does not dissolve in water, and does not react with acids. It decomposes in aqueous sodium hydroxide solution to release formaldehyde odor. The explosion point of TNEOF is 229 ° C, though it begins to decompose at 190 ° C. Its explosion heat is 6.3076 J/g and specific volume is 682 L/kg.[1] Its structure is closely related to that of trinitroethylorthocarbonate (TNEOC). Both are highly explosive and very shock-sensitive, and may be dissolved in nitroalkanes to reduce their shock-sensitivity.[1]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Tris(2,2,2-trinitroethyl)orthoformate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H7N9O21 | |
| Molar mass | 553.174 |
| Appearance | Colorless crystals |
| Melting point | 128 °C (262 °F; 401 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Liu, Jiping (2015). Liquid Explosives. Springer. pp. 5, 6, 8, 136, 309. ISBN 9783662458471. Retrieved 26 March 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
