Triphenylborane
Triphenylborane, often abbreviated to BPh3 where Ph is the phenyl group C6H5-, is a chemical compound with the formula B(C6H5)3. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Triphenylborane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.277 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H15B | |
| Molar mass | 242.12 g/mol |
| Appearance | White crystals |
| Melting point | 142 °C (288 °F; 415 K) |
| Boiling point | 203 °C (397 °F; 476 K) (15 mmHg) |
| Insoluble | |
| Structure | |
| trigonal planar | |
| Hazards | |
| R-phrases (outdated) | R11 |
| S-phrases (outdated) | S16 S22 S24/25 |
| Related compounds | |
Related isoelectronic |
Triphenylmethyl cation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
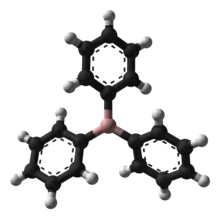
Structure and properties
The core of the compound, BC3, has a trigonal planar structure. The phenyl groups are rotated at about a 30° angle from the core plane.[1]
Even though triphenylborane and tris(pentafluorophenyl)borane are structurally similar, their Lewis acidity is not. BPh3 is a weak Lewis acid while B(C6F5)3 is a strong Lewis acid due to the electronegativity of the fluorine atoms. Other boron Lewis acids include BF3 and BCl3.[2]
Synthesis
Triphenylborane was first synthesized in 1922.[3] It is typically made with boron trifluoride diethyl etherate and the Grignard reagent, phenylmagnesium bromide.[4]
- BF3•O(C2H5)2 + 3 C6H5MgBr → B(C6H5)3 + 3 MgBrF + (C2H5)2O
Triphenylborane can also be synthesized on a smaller scale by the thermal decomposition of trimethylammonium tetraphenylborate.[5]
- [B(C6H5)4][NH(CH3)3] → B(C6H5)3 + N(CH3)3 + C6H6
Applications
Triphenylborane is made commercially by a process developed by Du Pont for use in its hydrocyanation of butadiene to adiponitrile, a nylon intermediate. Du Pont produces triphenylborane by reacting sodium metal, a haloaromatic (chlorobenzene), and a secondary alkyl borate ester.[6]
Triphenylborane can be used to make triarylborane amine complexes, such as pyridine-triphenylborane. Triarylborane amine complexes are used as catalysts for the polymerization of acrylic esters.[6]
References
- Zettler, F.; Hausen, H. D.; Hess, H. (1974). "Crystal and Molecular Structure of Triphenylborane". J. Organomet. Chem. 72 (2): 157. doi:10.1016/S0022-328X(00)81488-6.
- Erker, G. (2005). "Tris(pentafluorophenyl)borane: a special boron Lewis acid for special reactions". Dalton Trans. (11): 1883–90. doi:10.1039/b503688g. PMID 15909033.
- E. Krause & R. Nitsche (1922). "Darstellung von organischen Bor-Verbindungen mit Hilfe von Borfluorid, II.: Bortriphenyl und Phenyl-borsäure". Chemische Berichte. 55 (5): 1261. doi:10.1002/cber.19220550513.
- R. Köster; P. Binger & W. Fenzl (1974). Triphenylborane. Inorg. Synth. Inorganic Syntheses. 15. pp. 134–136. doi:10.1002/9780470132463.ch30. ISBN 978-0-470-13246-3.
- G. Wittig; P. Raff (1951). "Über Komplexbildung mit Triphenyl-bor". Liebigs Annalen der Chemie. 573: 195. doi:10.1002/jlac.19515730118.
- C. R. Guibert and J. L. Little, “Alkyl- and Arylboranes,” Ullmanns’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, 2005. doi:10.1002/14356007.a04_309