Triphenylmethyl hexafluorophosphate
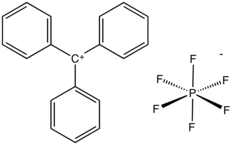
Triphenylmethyl hexafluorophosphate is an organic salt with the formula C
19H
15F
6P or (C
6H
5)
3CPF
6, consisting of the triphenylmethyl cation [(C
6H
5)
3C]+
and the hexafluorophosphate anion] [PF
6]−
. The cation is also called triphenylcarbenium, trityl cation, or tritylium.[1]
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
Trityl hexafluorophosphate Triphenylcarbenium hexafluorophosphate Tritylium hexafluorophosphate Diphenylmethylbenzene hexafluorophosphate (IUPAC) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.467 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H15F6P | |
| Molar mass | 388.31 g/mol |
| Appearance | brown powder |
| Melting point | 145 °C (293 °F; 418 K) |
| Hazards | |
| Safety data sheet | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triphenylmethyl hexafluorophosphate is a brown powder that hydrolyzes readily to triphenylmethanol. It is used as a catalyst and reagent in organic syntheses.[2]
Preparation
Triphenylmethyl hexafluorophosphate can be prepared by combining silver hexafluorophosphate with triphenylmethyl chloride:[3]
- AgPF6 + (C6H5)3CCl → (C6H5)3CPF6 + AgCl
A second method involves protonolysis of generating triphenylmethanol :[4]
- HPF6 + (C6H5)3COH → (C6H5)3CPF6 + H2O
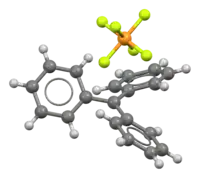
Structure and reactions
Triphenylmethyl hexafluorophosphate readily hydrolyzes, in a reaction that is the reverse of one of its syntheses:[5]
- (C6H5)3CPF6 + H2O → (C6H5)3COH + HPF6
Triphenylmethyl hexafluorophosphate has been used for abstracting hydride (H−
) from organic compounds. Treatment of metal-alkene and diene complexes one can generate allyl and pentadienyl complexes, respectively.[2]
Triphenylmethyl perchlorate is a common substitute for triphenylmethyl hexafluorophosphate. However, the perchlorate is not used as widely, because, like other organic perchlorates, it is potentially explosive.[2]
References
- Triphenylcarbenium hexafluorophosphate from PubChem
- Urch, C. (2001). "Triphenylmethyl Hexafluorophosphate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt363f. ISBN 0471936235.
- Sharp, D.; Shepard, N. (1956). "Complex Fluorides. Part VIII". University Chemical Laboratory, Cambridge: 674–682.
- Olah, G.; Svoboda, J.; Olah, J. (1972). "Preparative Carbocation Chemistry; IV. Improved Preparation of Triphenylcarbenium (Trityl) Salts". Synthesis. 1972 (10): 544. doi:10.1055/s-1972-21914.
- Fernandez-Galan, R.; Manzano, B; Otero, A; Lanfranchi, M; Pellinghelli, M. (1994). "19F and 31P NMR Evidence for Silver Hexafluorophosphate Hydrolysis in Solution". Inorg. Chem. 33 (10): 2309–2312. doi:10.1021/ic00088a039.