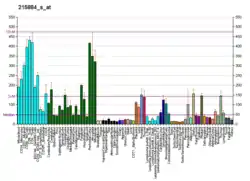
UBQLN2
Ubiquilin-2 is a protein that in humans is encoded by the UBQLN2 gene.[5][6]
Function
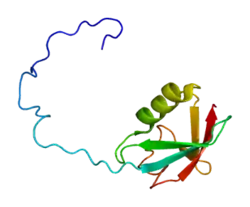
This gene encodes a ubiquitin-like protein (ubiquilin) that shares high degree of similarity with related products in yeast, rat and frog. Ubiquilins contain a N-terminal ubiquitin-like domain and a C-terminal ubiquitin-associated domain. They physically associate with both proteasomes and ubiquitin ligases, and are thus thought to functionally link the ubiquitination machinery to the proteasome to effect in vivo protein degradation. This ubiquilin has also been shown to bind the ATPase domain of the Hsp70-like Stch protein.[6]
Similarity to other proteins
Human UBQLN2 shares a high degree of similarity with related ubiquilins including UBQLN1 and UBQLN4.[7]
Clinical significance
In a small proportion of familial amyotrophic lateral sclerosis (fALS), the UBQLN2 gene is mutated, causing formation of a non-functional Ubiquilin 2 enzyme. This non-functioning enzyme leads to the accumulation of ubiquinated proteins in the lower motor neurons and upper corticospinal motor neurons, due to the fact that ubiquilin 2 normally degrades these ubiquinated proteins, but cannot if the ALS mutation is present.[8] The same accumulations occur in patients without UBQLN2 mutations, but with mutations in other genes, including TDP-43 and C9ORF72.
References
- GRCh38: Ensembl release 89: ENSG00000188021 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000050148 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Kaye FJ, Modi S, Ivanovska I, Koonin EV, Thress K, Kubo A, Kornbluth S, Rose MD (Mar 2000). "A family of ubiquitin-like proteins binds the ATPase domain of Hsp70-like Stch" (PDF). FEBS Lett. 467 (2–3): 348–55. doi:10.1016/S0014-5793(00)01135-2. PMID 10675567.
- "Entrez Gene: UBQLN2 ubiquilin 2".
- Marín I (March 2014). "The ubiquilin gene family: evolutionary patterns and functional insights". BMC Evol Biol. 14: 63. doi:10.1186/1471-2148-14-63. PMC 4230246. PMID 24674348.
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T (August 2011). "Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia". Nature. 477 (7363): 211–215. doi:10.1038/nature10353. PMC 3169705. PMID 21857683. Lay summary – New York Times.
- Kim TY, Kim E, Yoon SK, Yoon JB (May 2008). "Herp enhances ER-associated protein degradation by recruiting ubiquilins". Biochem. Biophys. Res. Commun. 369 (2): 741–6. doi:10.1016/j.bbrc.2008.02.086. PMID 18307982.
- Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, Gill G, Howley PM (August 2000). "The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome". Mol. Cell. 6 (2): 409–19. doi:10.1016/S1097-2765(00)00040-X. PMID 10983987.
Further reading
- Ueki N, Oda T, Kondo M, et al. (1999). "Selection system for genes encoding nuclear-targeted proteins". Nat. Biotechnol. 16 (13): 1338–42. doi:10.1038/4315. PMID 9853615.
- Kaye FJ, Shows TB (2000). "Assignment of ubiquilin2 (UBQLN2) to human chromosome xp11. 23→p11.1 by GeneBridge radiation hybrids". Cytogenet. Cell Genet. 89 (1–2): 116–7. doi:10.1159/000015588. PMID 10894951.
- Kleijnen MF, Shih AH, Zhou P, et al. (2000). "The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome". Mol. Cell. 6 (2): 409–19. doi:10.1016/S1097-2765(00)00040-X. PMID 10983987.
- Murillas R, Simms KS, Hatakeyama S, et al. (2002). "Identification of developmentally expressed proteins that functionally interact with Nedd4 ubiquitin ligase". J. Biol. Chem. 277 (4): 2897–907. doi:10.1074/jbc.M110047200. PMID 11717310.
- Walters KJ, Kleijnen MF, Goh AM, et al. (2002). "Structural studies of the interaction between ubiquitin family proteins and proteasome subunit S5a". Biochemistry. 41 (6): 1767–77. doi:10.1021/bi011892y. PMID 11827521.
- Saeki Y, Sone T, Toh-e A, Yokosawa H (2002). "Identification of ubiquitin-like protein-binding subunits of the 26S proteasome". Biochem. Biophys. Res. Commun. 296 (4): 813–9. doi:10.1016/S0006-291X(02)02002-8. PMID 12200120.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Kleijnen MF, Alarcon RM, Howley PM (2004). "The ubiquitin-associated domain of hPLIC-2 interacts with the proteasome". Mol. Biol. Cell. 14 (9): 3868–75. doi:10.1091/mbc.E02-11-0766. PMC 196580. PMID 12972570.
- Colland F, Jacq X, Trouplin V, et al. (2004). "Functional proteomics mapping of a human signaling pathway". Genome Res. 14 (7): 1324–32. doi:10.1101/gr.2334104. PMC 442148. PMID 15231748.
- Ross MT, Grafham DV, Coffey AJ, et al. (2005). "The DNA sequence of the human X chromosome". Nature. 434 (7031): 325–37. doi:10.1038/nature03440. PMC 2665286. PMID 15772651.
- Massey LK, Mah AL, Monteiro MJ (2006). "Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin". Biochem. J. 391 (Pt 3): 513–25. doi:10.1042/BJ20050491. PMC 1276952. PMID 15975090.
- Lim J, Hao T, Shaw C, et al. (2006). "A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration". Cell. 125 (4): 801–14. doi:10.1016/j.cell.2006.03.032. PMID 16713569.
- Ford DL, Monteiro MJ (2006). "Dimerization of ubiquilin is dependent upon the central region of the protein: evidence that the monomer, but not the dimer, is involved in binding presenilins". Biochem. J. 399 (3): 397–404. doi:10.1042/BJ20060441. PMC 1615901. PMID 16813565.
- Kang Y, Zhang N, Koepp DM, Walters KJ (2007). "Ubiquitin receptor proteins hHR23a and hPLIC2 interact". J. Mol. Biol. 365 (4): 1093–101. doi:10.1016/j.jmb.2006.10.056. PMC 1994665. PMID 17098253.