Uronic acid
Uronic acids (/ʊˈrɒnɪk/) are a class of sugar acids with both carbonyl and carboxylic acid functional groups. They are sugars in which the terminal carbon's hydroxyl group has been oxidized to a carboxylic acid. Oxidation of the terminal aldehyde instead yields an aldonic acid, while oxidation of both the terminal hydroxyl group and the aldehyde yields an aldaric acid. The names of uronic acids are generally based on their parent sugars, for example, the uronic acid analog of glucose is glucuronic acid. Uronic acids derived from hexoses are known as hexuronic acids and uronic acids derived from pentoses are known as penturonic acids.[1]
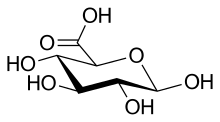

Examples
Some of these compounds have important biochemical functions; for example, many wastes in the human body are excreted in the urine as their glucuronate salts, and iduronic acid is a component of some structural complexes such as proteoglycans.
References
- "Hexuronic acid". encyclopedia.com.
External links
- Uronic+Acids at the US National Library of Medicine Medical Subject Headings (MeSH)
- Synthesis at chembio.uoguelph.ca