Vinylsilane
Vinylsilane refers to an organosilicon compound with chemical formula CH2=CHSiH3. It is a derivative of silane (SiH4). The compound, which is a colorless gas, is mainly of theoretical interest.[1]
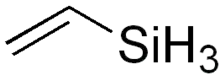 | |
| Names | |
|---|---|
| Other names
ethenylsilane, vinyl silane | |
| Identifiers | |
| ECHA InfoCard | 100.027.926 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| C2H6Si | |
| Molar mass | 58.155 g·mol−1 |
| Appearance | colorless gas |
| Boiling point | -22.8 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Substituted vinylsilanes
More commonly used than the parent vinylsilane are vinyl-substituted silanes with other substituents on silicon. In the area of organic synthesis, vinylsilanes are useful intermediates.[2]
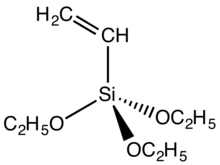
Vinyltriethoxysilane is a common vinylsilane.
In the area of polymer chemistry and materials science, vinyltrimethoxysilane or vinyltriethoxysilane serve as monomers and coupling agents.
Preparation
Vinylsilanes are often prepared by hydrosilylation of alkynes. They can be made by the reaction of alkenyl lithium and Grignard reagents with chlorosilanes. In some cases dehydrogenative silylation is another method.[3]
References
- Ring, M. A.; O'Neal, H. E.; Rickborn, S. F.; Sawrey, B. A. (1983). "Kinetics of the high-temperature thermal decomposition of silanes and alkylsilanes". Organometallics. 2: 1891–4. doi:10.1021/om50006a038.CS1 maint: uses authors parameter (link)
- Fleming, Ian; Dunogues, Jacques; Smithers, Roger (1989). "The electrophilic substitution of allylsilanes and vinylsilanes". Organic Reactions. 37: 57–575. doi:10.1002/0471264180.or037.02.CS1 maint: uses authors parameter (link)
- Lu, B.; Falck, J. R. (2010). "Iridium-Catalyzed (Z)-Trialkylsilylation of Terminal Olefins". J. Org. Chem. 75: 1701–1705. doi:10.1021/jo902678p. PMC 2830331. PMID 20136153.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
