XMEN disease
XMEN disease is a rare genetic disorder of the immune system that illustrates the role of Mg2+ in cell signaling. XMEN stands for “X-linked immunodeficiency with magnesium defect, Epstein-Barr virus (EBV) infection, and neoplasia.” The disease is characterized by CD4 lymphopenia, severe chronic viral infections, and defective T-lymphocyte activation. Investigators in the laboratory of Dr. Michael Lenardo, National Institute of Allergy and Infectious Diseases at the National Institutes of Health first described this condition in 2011.[1][2][3]
| XMEN disease | |
|---|---|
| Other names | Combined immunodeficiency due to MAGT1 deficiency |
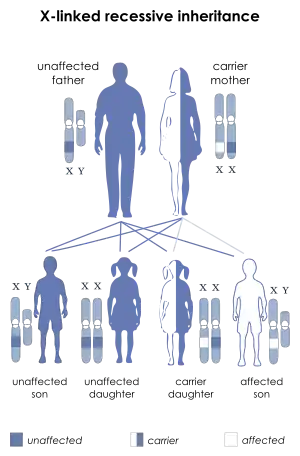 | |
| X-linked recessive is the manner in which this condition is inherited | |
Presentation
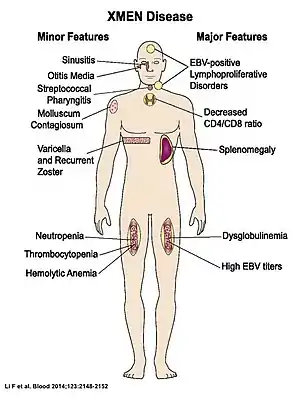
XMEN patients have splenomegaly, chronic Epstein Barr Virus (EBV) infection, and are developmentally normal. They have an increased susceptibility for developing EBV+ lymphoma. Additionally, XMEN patients have excessive infections consistent with the underlying immunodeficiency. These infections included recurrent otitis media, sinusitis, viral pneumonia, diarrhea, upper respiratory infections, epiglottitis, and pertussis. Although autoimmune symptoms do not feature prominently in XMEN autoimmune cytopenias were observed in two unrelated patients.[1]
In the figure to the left, major features are present in all XMEN patients, while minor features are found only in some.
Genetics
XMEN disease is caused by loss of function mutations in the gene MAGT1.[1] MAGT1 is a 70 kb gene with 10 exons encoding for a 335 amino acid protein that maps to Xq21.1.[4] MagT1 is a component of the N-linked glycosylation machinery (oligosacchyrltransferase, OST), a fundamental component of all cells that regulates the attachment of sugar moieties onto specific sites in proteins. Many of the immunodeficiency-related features of XMEN disease are related to the hypoglycosylation phenotype caused by loss of the OST component MagT1.[5][6]
The conclusive connection between MAGT1, glycosylation, and Mg2+ import remains unknown. A prominent hypothesis from XMEN patients suggests that MAGT1's role in glycosylation is essential to the function (either directly or indirectly) of a Mg+2 transporter.[5][6]
MAGT1 is evolutionarily conserved and expressed in all mammalian cells with higher expression in hematopoietic lineages.[7] XMEN patients have been found to carry both MAGT1 deletions and missense mutations. However, the severity of the phenotype is not entirely explained by the genotype. The disease severity also likely depends on environmental and other genetic factors.[3]
XMEN disease follows an X-linked inheritance pattern because the MAGT1 is located on the X chromosome. Mothers of XMEN patients exhibit preferential X chromosome inactivation of the chromosome with the mutation in their hematopoietic cells and are asymptomatic.
Diagnosis
XMEN patients generally have chronically high levels of EBV with increased EBV-infected cells, diminished thymic output of CD4+ cells, reduced CD4:CD8 ratio, moderately high B cell counts, and mild neutropenia. Their neutropenia may be related to their chronic EBV. Some patients also showed defective T cell proliferation in response to mitogen stimulation, variable immunoglobulin deficiencies, or deficient vaccination response.[1][2][3]
Treatment
Once a diagnosis is made, each individual's treatment is based on an individual’s clinical condition. Hematopoietic stem cell transplant is a possible treatment of this condition but its effectiveness is unproven.
Additionally, magnesium supplementation is a promising potential treatment for XMEN. One of the consequences of loss of MAGT1 function is a decreased level of unbound intracellular Mg2+. This decrease leads to loss of expression of an immune cell receptor called NKG2D, which is involved in EBV-immunity. Remarkably, Mg2+ supplementation can restore NKG2D expression and other functions that are abnormal in patients with XMEN disease. Early evidence suggests continuous oral magnesium threonate supplementation is safe and well tolerated. Nonetheless, further research is needed to evaluate the use of Mg2+ as a treatment for XMEN. It remains unclear if such supplementation will protect against the development of lymphoma in XMEN patients.[3] Investigators at the National Institute of Allergy and Infectious Diseases at the US National Institutes of Health currently have clinical protocols to study new approaches to the diagnosis and treatment of this disorder.[8][9]
References
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. (July 2011). "Second messenger role for Mg2+ revealed by human T-cell immunodeficiency". Nature. 475 (7357): 471–6. doi:10.1038/nature10246. PMC 3159560. PMID 21796205.
- Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. (July 2013). "Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D". Science. 341 (6142): 186–91. Bibcode:2013Sci...341..186C. doi:10.1126/science.1240094. PMC 3894782. PMID 23846901.
- Li FY, Chaigne-Delalande B, Su H, Uzel G, Matthews H, Lenardo MJ (April 2014). "XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus". Blood. 123 (14): 2148–52. doi:10.1182/blood-2013-11-538686. PMC 3975255. PMID 24550228.
- Goytain A, Quamme GA (April 2005). "Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties". BMC Genomics. 6 (48): 48. doi:10.1186/1471-2164-6-48. PMC 1129089. PMID 15804357.
- Matsuda-Lennikov M, Biancalana M, Zou J, Ravell JC, Zheng L, Kanellopoulou C, et al. (September 2019). "N-linked glycosylation and expression of immune-response genes". The Journal of Biological Chemistry. 294 (37): 13638–13656. doi:10.1074/jbc.RA119.008903. PMC 6746436. PMID 31337704.
- Ravell JC, Matsuda-Lennikov M, Chauvin SD, Zou J, Biancalana M, Deeb SJ, et al. (January 2020). "Defective glycosylation and multisystem abnormalities characterize the primary immunodeficiency XMEN disease". The Journal of Clinical Investigation. 130 (1): 507–522. doi:10.1172/JCI131116. PMC 6934229. PMID 31714901.
- Zhou H, Clapham DE (September 2009). "Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development". Proceedings of the National Academy of Sciences of the United States of America. 106 (37): 15750–5. Bibcode:2009PNAS..10615750Z. doi:10.1073/pnas.0908332106. PMC 2732712. PMID 19717468.
- Clinical trial number NCT00001467 for "Genetic Analysis of Immune Disorders" at ClinicalTrials.gov
- Clinical trial number NCT00246857 for "Screening Protocol for Genetic Diseases of Lymphocyte Homeostasis and Programmed Cell Death" at ClinicalTrials.gov