Genotype
A genotype is an organism’s complete set of genetic material.[1] Often though, genotype is used to refer to a single gene or set of genes, such as the genotype for eye color. The genes take part in determining the characteristics that are observable (phenotype) in an organism, such as hair color, height, etc.[2] An example of a characteristic determined by a genotype is the petal color in a pea plant. The collection of all genetic possibilities for a single trait are called alleles; two alleles for petal color are purple and white.[3]
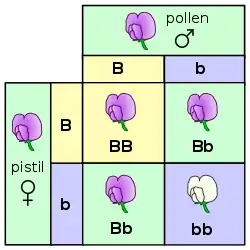
The genotype is one of three factors that determine phenotype. The other two are the environmental (not inherited) and the epigenetic (inherited) factors. Not all individuals with the same genotype look or act the same way because appearance and behavior are modified by environmental and growing conditions. Likewise, not all organisms that look alike necessarily have the same genotype. One would typically refer to an individual's genotype with regard to a particular gene of interest and the combination of alleles the individual carries (see homozygous, heterozygous).[4] Genotypes are often denoted with letters, for example Bb, where B stands for one allele and b for another.
Somatic mutations that are acquired rather than inherited, such as those in cancers, are not part of the individual's genotype. Hence, scientists and physicians sometimes talk about the genotype of a particular cancer, that is, of the disease as distinct from the diseased.
The term genotype was coined by the Danish botanist Wilhelm Johannsen in 1903.[5]
Phenotype
Any given gene will usually cause an observable change in an organism, known as the phenotype. The terms genotype and phenotype are distinct for at least two reasons:
- To distinguish the source of an observer's knowledge (one can know about genotype by observing DNA; one can know about phenotype by observing outward appearance of an organism).
- Genotype and phenotype are not always directly correlated. Some genes only express a given phenotype in certain environmental conditions. Conversely, some phenotypes could be the result of multiple genotypes. The genotype is commonly mixed up with the phenotype which describes the end result of both the genetic and the environmental factors giving the observed expression (e.g. blue eyes, hair color, or various hereditary diseases).
A simple example to illustrate genotype as distinct from phenotype is the flower colour in pea plants (see Gregor Mendel). There are three available genotypes, PP (homozygous dominant ), Pp (heterozygous), and pp (homozygous recessive). All three have different genotypes but the first two have the same phenotype (purple) as distinct from the third (white).
A more technical example to illustrate genotype is the single-nucleotide polymorphism or SNP. A SNP occurs when corresponding sequences of DNA from different individuals differ at one DNA base, for example where the sequence AAGCCTA changes to AAGCTTA.[6] This contains two alleles : C and T. SNPs typically have three genotypes, denoted generically AA Aa and aa. In the example above, the three genotypes would be CC, CT and TT. Other types of genetic marker, such as microsatellites, can have more than two alleles, and thus many different genotypes.
Penetrance is the proportion of individuals showing a specified genotype in their phenotype under a given set of environmental conditions.[7]
Mendelian inheritance
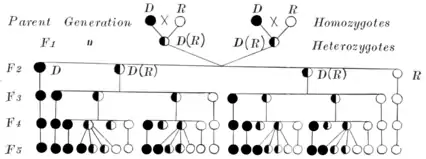
The distinction between genotype and phenotype is commonly experienced when studying family patterns for certain hereditary diseases or conditions, for example, hemophilia. Humans and most animals are diploid; thus there are two alleles for any given gene. These alleles can be the same (homozygous) or different (heterozygous), depending on the individual (see zygote). With a dominant allele, such as having dark hair, the offspring is guaranteed to exhibit the trait in question irrespective of the second allele.
In the case of an albino with a recessive allele (aa), the phenotype depends upon the other allele (Aa, aA, aa or AA). An affected person mating with a heterozygous individual (Aa or aA, also carrier) there is a 50-50 chance the offspring will be albino's phenotype. If a heterozygote mates with another heterozygote, there is 75% chance passing the gene on and only a 25% chance that the gene will be displayed. A homozygous dominant (AA) individual has a normal phenotype and no risk of abnormal offspring. A homozygous recessive individual has an abnormal phenotype and is guaranteed to pass the abnormal gene onto offspring.
Non-Mendelian Inheritance
Sex-Linked Traits
In the case of hemophilia,[8] colorblindness,[9] or other sex-linked traits, the gene is only carried on the X chromosome. Therefore, only individuals with two X chromosomes can be a carrier in which the abnormality is not displayed. This person has a normal phenotype, but runs a 50-50 chance, with an unaffected partner, of passing their abnormal gene on to her offspring. If she mated with a man with haemophilia (another carrier) there would be a 75% chance of passing on the gene.
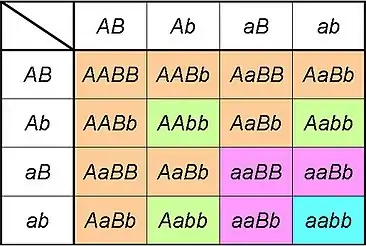
Traits involving multiple genes
Certain phenotypes do not follow the same patterns as determined by Mendelian genetics. This is often due to the final phenotype being determined by multiple genes. The resulting phenotype of these related genes are essentially a combination of the individual genes, creating an even greater variety. Being connected to multiple genes dramatically increases the number of possible genotypes for the trait. With the examples found in Mendelian genetics, each trait had one gene, with two possible inherited alleles, and 3 possible combinations of those alleles. If each gene still only has two alleles, the genotype for a trait involving 2 would now have nine possible genotypes. For example, you may have one gene expressed with "A" for the dominant allele and "a" for the recessive allele, and the other gene using "B" and "b" in the same way. The possible genotypes for this trait are AABB, AaBB, aaBB, AABb, AaBb, aaBb, aaBB, aaBb, and aabb. Below we will discuss a few ways genes can interact to contribute to a single trait
Epistasis
Epistasis is when the phenotype of one gene is affected by one or more other genes.[10] This is often through some sort of masking effect of one gene on the other.[11] For example, the "A" gene codes for hair color, a dominant "A" allele codes for brown hair, and a recessive "a" allele codes for blonde hair, but a separate "B" gene controls hair growth, and a recessive "b" allele causes baldness. If the individual has the BB or Bb genotype, then they produce hair and the hair color phenotype can be observed, but if the individual has a bb genotype, then the person is bald which masks the A gene entirely.
Polygenic Traits
A polygenic trait is one whose phenotype is dependent on the additive effects of multiple genes. The contributions of each of these genes are typically small and add up to a final phenotype with a large amount of variation. A well studied example of this is the number of sensory bristles on a fly.[12] These types of additive effects is also the explanation for the amount of variation in human eye color.
Determination
Genotyping is the process of elucidating the genotype of an individual with a biological assay. Also known as a genotypic assay, techniques include PCR, DNA fragment analysis, allele specific oligonucleotide (ASO) probes, DNA sequencing, and nucleic acid hybridization to DNA microarrays or beads. Several common genotyping techniques include restriction fragment length polymorphism (RFLP), terminal restriction fragment length polymorphism (t-RFLP),[13] amplified fragment length polymorphism (AFLP),[14] and multiplex ligation-dependent probe amplification (MLPA).[15]
DNA fragment analysis can also be used to determine disease-causing genetic aberrations such as microsatellite instability (MSI),[16] trisomy[17] or aneuploidy, and loss of heterozygosity (LOH).[18] MSI and LOH in particular have been associated with cancer cell genotypes for colon,[19] breast[20] and cervical cancer.[21]
The most common chromosomal aneuploidy is a trisomy of chromosome 21, which manifests itself as Down syndrome. Current technological limitations typically allow only a fraction of an individual's genotype to be determined efficiently.
See also
References
- "What is genotype? What is phenotype? – pgEd". pged.org. Retrieved 2020-06-22.
- Pierce, Benjamin (2020). Genetics A Conceptual Approach. NY, New York: Macmillian. ISBN 978-1-319-29714-5.
- Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2014). Essential Cell Biology (4th ed.). New York, NY: Garland Science. p. 659. ISBN 978-0-8153-4454-4.
- Griffiths AJ, Gelbart WM, Miller JH, et al. (1999). "Genetics begins with Variation". Modern Genetic Analysis. New York: W. H. Freeman.
- Johannsen W (1903). "Om arvelighed i samfund og i rene linier". Oversigt Birdy over Det Kongelige Danske Videnskabernes Selskabs Forhandlingerm (in Danish). 3: 247–70. German ed. "Erblichkeit in Populationen und in reinen Linien" (in German). Jena: Gustav Fischer. 1903.. Also see his monograph Johannsen W (1905). Arvelighedslærens elementer horse [The Elements of Heredity] (in Danish). Copenhagen. which was rewritten, enlarged and translated into German as Johannsen W (1905). Elemente der exakten Erblichkeitslehre (in German). Jena: Gustav Fischer.
- Vallente, R. U., PhD. (2020). Single Nucleotide Polymorphism. Salem Press Encyclopedia of Science.
- Allaby, Michael, ed. (2009). A dictionary of zoology (3rd ed.). Oxford: Oxford University Press. ISBN 9780199233410. OCLC 260204631.
- Ulutin ON, Müftüoğlu A, Palamar S (September 1965). "Haemophilia A in a girl with female sex-chromatin pattern". Thrombosis et Diathesis Haemorrhagica. 14 (1–2): 65–73. PMID 16955966.
- Jackson, C. E.; Symon, W. E.; Mann, J. D. (December 1964). "X Chromosome Mapping of Genes for Red-Green Colorblindness and Xg". American Journal of Human Genetics. 16: 403–409. ISSN 0002-9297. PMC 1932325. PMID 14250421.
- Gros, Pierre-Alexis; Nagard, Hervé Le; Tenaillon, Olivier (2009-05-01). "The Evolution of Epistasis and Its Links With Genetic Robustness, Complexity and Drift in a Phenotypic Model of Adaptation". Genetics. 182 (1): 277–293. doi:10.1534/genetics.108.099127. ISSN 0016-6731. PMC 2674823. PMID 19279327.
- Rieger, Rigomar. (1976). Glossary of genetics and cytogenetics : classical and molecular. Michaelis, Arnd,, Green, Melvin M. (4th completely rev. ed.). Berlin: Springer-Verlag. ISBN 0-387-07668-9. OCLC 2202589.
- Mackay, T. F. (December 1995). "The genetic basis of quantitative variation: numbers of sensory bristles of Drosophila melanogaster as a model system". Trends in Genetics. 11 (12): 464–470. doi:10.1016/s0168-9525(00)89154-4. ISSN 0168-9525. PMID 8533161.
- Hulce D, Liu C (July 2006). "SoftGenetics Application Note - GeneMarker® Software for Terminal-Restriction Fragment Length Polymorphism (T-RFLP) Data Analysis" (PDF). SoftGenetics. Archived from the original (PDF) on 2007-06-13.
- "Keygene.com Homepage" Archived 2011-06-28 at the Wayback Machine
- "SoftGenetics Application Note - Software for Multiplex Ligation-dependent Probe Amplification (MLPA™)" (PDF). SoftGenetics. April 2006. Archived from the original (PDF) on 2011-07-16. Retrieved 2011-03-13.
- He H, Ning W, Liu J (March 2007). "SoftGenetics Application Note - Microsatellite Instability Analysis with GeneMarker® Tamela Serensits" (PDF). SoftGenetics. Archived from the original (PDF) on 2007-09-23.
- "SoftGenetics Application Note - GeneMarker® Software for Trisomy Analysis" (PDF). SoftGenetics. November 2006. Archived from the original (PDF) on 2007-07-28.
- Serensits P, He H, Ning W, Liu J (March 2007). "SoftGenetics Application Note - Loss of Heterozygosity Detection with GeneMarker" (PDF). SoftGenetics. Archived from the original (PDF) on 2007-07-28.
- Boland CR, Goel A (June 2010). "Microsatellite instability in colorectal cancer". Gastroenterology. 138 (6): 2073–2087.e3. doi:10.1053/j.gastro.2009.12.064. PMC 3037515. PMID 20420947.
- Kurata K, Kubo M, Kai M, Mori H, Kawaji H, Kaneshiro K, et al. (January 2020). "Microsatellite instability in Japanese female patients with triple-negative breast cancer". Breast Cancer. 27 (3): 490–498. doi:10.1007/s12282-019-01043-5. PMC 7196096. PMID 31907878.
- Chambuso R, Kaambo E, Denny L, Gray CM, Williamson AL, Migdalska-Sęk M, et al. (2019-10-15). "HLA II Locus in HIV-1/HPV Co-infected Women". Frontiers in Oncology. 9: 951. doi:10.3389/fonc.2019.00951. PMC 6803484. PMID 31681558.
External links
| Look up genotype, phenotype, inheritance, or genome in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Genotypes. |