Zabofloxacin
Zabofloxacin is an investigational fluoroquinolone antibiotic for multidrug-resistant infections due to Gram-positive bacteria.[1][2][3] It also has activity against Neisseria gonorrhoeae including strains that are resistant to other quinolone antibiotics.[4]
 | |
| Names | |
|---|---|
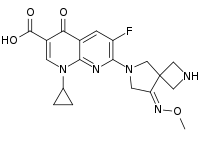
| IUPAC name
1-Cyclopropyl-6-fluoro-7-[(8E)-8-(methoxyimino)-2,6-diazaspiro[3.4]oct-6-yl]-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid | |
| Other names
DW-224a | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H20FN5O4 | |
| Molar mass | 401.392 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zabofloxacin was discovered by Dong Wha Pharmaceuticals and licensed to Pacific Beach BioSciences for development.[5]
References
- HyeKyung Han; Sung Eun Kim; Kwang-Hee Shin; Cheolhee Lim; Kyoung Soo Lim; Kyung-Sang Yu; Joo-Youn Cho (October 2013). "Comparison of pharmacokinetics between new quinolone antibiotics: the zabofloxacin hydrochloride capsule and the zabofloxacin aspartate tablet". Current Medical Research and Opinion. 29 (10): 1349–1355. doi:10.1185/03007995.2013.825591.
- Jong-Hwa Lee; Jung-Heun Ha; Dae-Hun Park; Dong-Rack Choi; Gye-Won Lee; Sung-Hoon Ahn & Choong-Yong Kim (2014). "Quantification Of Zabofloxacin In Rat Plasma Using Hplc-Uv Detector And Its Application To A Pharmacokinetic Study". Journal of Liquid Chromatography & Related Technologies. 37 (3): 311–320. doi:10.1080/10826076.2012.745137.
- Kim EJ, Shin WH, Kim KS, Han SS (Nov 2004). "Safety pharmacology of DW-224a, a novel fluoroquinolone antibiotic agent". Drug Chem Toxicol. 27 (4): 295–307. doi:10.1081/DCT-200039708. PMID 15573468.
- Jones RN, Biedenbach DJ, Ambrose PG, Wikler MA (2008). "Zabofloxacin (DW-224a) activity against Neisseria gonorrhoeae including quinolone-resistant strains". Diagn Microbiol Infect Dis. 62 (1): 110–112. doi:10.1016/j.diagmicrobio.2008.05.010. PMID 18620833.
- "Pacific Beach BioSciences and Dong Wha Pharmaceuticals Sign Exclusive License Agreement to Develop and Commercialize Zabofloxacin (PB-101, DW-224a)". GlobeNewswire. August 10, 2007.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
