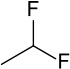
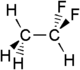
1,1-Difluoroethane
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years).[2][3]
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1-Difluoroethane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.788 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4F2 | |||
| Molar mass | 66.05 g/mol | ||
| Density | 900 g/L @ 25 °C | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | −25 °C (−13 °F; 248 K) | ||
| 0.54% @ 0 °C | |||
| Vapor pressure |
| ||
| Viscosity | 8.87 µPa·s (0.00887 cP) @ 25 °C | ||
| Hazards | |||
| Safety data sheet | SDS for 1,1-difluoroethane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
1,1-Difluoroethane is produced by the mercury-catalyzed addition of hydrogen fluoride to acetylene:[4]
- HCCH + 2 HF → CH3CHF2
The intermediate in this process is vinyl fluoride (C2H3F), the monomeric precursor to polyvinyl fluoride.
Uses
With a relatively low global warming potential (GWP) index of 124 and favorable thermophysical properties, 1,1-difluoroethane has been proposed as an environmentally friendly alternative to R134a. Despite its flammability, it also presents operating pressures and volumetric cooling capacity (VCC) similar to R134a so it can be used in large chillers[5] or in more particular applications like heat pipe finned heat exchangers.[6]
Furthermore, 1,1-difluoroethane is also commonly used in gas dusters and many consumer aerosol products, especially those subject to stringent volatile organic compound (VOC) requirements.
The molecular weight of difluoroethane is 66, making it a useful and convenient tool for detecting vacuum leaks in GC-MS systems. The cheap and freely available gas has a molecular weight and fragmentation pattern (base peak 51 m/z in typical EI-MS, major peak at 65 m/z) distinct from anything in air. If mass peaks corresponding to 1,1-difluoroethane are observed immediately after spraying a suspect leak point, leaks may be identified.
Safety
Difluoroethane is an intoxicant and precipitates fatal cardiac arrhythmia.[7] Several reports of fatal car crashes have been linked to drivers huffing 1,1-difluoroethane.[8][9][10] Actress Skye McCole Bartusiak died due to combined effects of difluoroethane and other drugs.[11] Because of inhalant abuse, a bitterant is added to some brands; however even this measure is not legally required and has not prevented widespread use of this product as a drug.
In a DuPont study, rats were exposed to up to 25,000 ppm (67,485 mg/m3) for six hours daily, five days a week for two years. This has become the no-observed-adverse-effect level for this substance. Prolonged exposure to 1,1-difluoroethane has been linked in humans to the development of coronary disease and angina.[12]
See also
References
- 1,1-Difluoroethane at Sigma-Aldrich
- "Changes in Atmospheric Constituents and in Radiative Forcing" (PDF). Cambridge University Press. 2007. p. 212. Retrieved 11 May 2017. dead link 18 February 2019
- "Global Warming Potentials of ODS Substitutes". U.S. Environmental Protection Agency. 2010. Archived from the original on 16 October 2010. Retrieved 20 September 2010.
- Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2010). "Fluorine Compounds, Organic". In Bohnet, Matthias; Bellussi, Giuseppe; Bus, James; et al. (eds.). Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons. doi:10.1002/14356007.a11_349.
- Longo, Giovanni A.; Zilio, Claudio; Righetti, Giulia (2015). "Condensation of the low GWP refrigerant HFC152a inside a Brazed Plate Heat Exchanger". Experimental Thermal and Fluid Science. 68: 509–515. doi:10.1016/j.expthermflusci.2015.06.010.
- Righetti, Giulia; Zilio, Claudio; Mancin, Simone; Longo, Giovanni A. (2018). "Heat Pipe Finned Heat Exchanger for Heat Recovery: Experimental Results and Modeling". Heat Transfer Engineering. 39 (12): 1011–1023. Bibcode:2018HTrEn..39.1011R. doi:10.1080/01457632.2017.1358483.
- Avella J, Wilson JC, Lehrer M (March 2006). "Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE)". The American Journal of Forensic Medicine and Pathology. 27 (1): 58–60. doi:10.1097/01.paf.0000202715.71009.0e. PMID 16501351.
- Broussard LA, Brustowicz T, Pittman T, Atkins KD, Presley L (November 1997). "Two traffic fatalities related to the use of difluoroethane". Journal of Forensic Sciences. 42 (6): 1186–7. PMID 9397568.
- Hahn, T; Avella, J; Lehrer, M (2006). "A motor vehicle accident fatality involving the inhalation of 1,1-difluoroethane". Journal of Analytical Toxicology. 30 (8): 638–42. doi:10.1093/jat/30.8.638. PMID 17132266.
- "Autopsy: man in crash died from inhaling computer cleaner". The Times News. 10 March 2012. Archived from the original on 12 March 2012.
- Duke, Alan (22 July 2014). "'Patriot' actress Skye McCole Bartusiak dead at 21". CNN Entertainment. Retrieved 24 February 2019.
- "1,1-Difluoroethane". National Library of Medicine HSDB Database. 1994. Retrieved 8 June 2010.